![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
181 Cards in this Set
- Front
- Back
|
What is the term for programmed cell death?
|
Apoptosis
|
|
|
What does apoptosis require?
|
- ATP
- Activation of cytosolic caspases |
|
|
What are the morphologic characteristics of cells undergoing apoptosis?
|
- Eosinophilic cytoplasm
- Cell shrinkage - Nuclear shrinkage (pyknosis) and basophilia - Membrane blebbing - Nuclear fragmentation (karyorrhexis) - Formation of apoptotic bodies (which are phagocytosed) |
|
|
What is the term for nuclear shrinkage?
|
Pyknosis
|
|
|
What is the term for nuclear fragmentation?
|
Karyorrhexis
|
|
|
What is a sensitive indicator of apoptosis?
|
DNA laddering
- During karyorrhexis (nuclear fragmentation), endonucleases cleave at internucleosomal regions - Yields 180-bp fragments |
|
|
How does radiation therapy cause apoptosis? What cells are affected?
|
- Radiation causes apoptosis of tumors and surrounding tissue via free radical formation and dsDNA breakage
- Rapidly dividing cells (eg, skin and GI mucosa) are very susceptible to radiation therapy induced apoptosis |
|
|
What are the pathways that cause apoptosis?
|
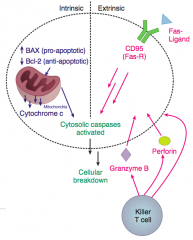
- Intrinsic pathway
- Extrinsic pathway: ligand receptor interactions (Fas-FasL) and immune cell (cytotoxic T-cell release of perforin and granzyme B) |
|
|
What is the purpose of the intrinsic pathway of apoptosis? When is it used?
|
Involved in tissue remodeling in embryogenesis
- Occurs when a regulating factor is withdrawn from a proliferating cell population (eg, ↓ IL-2 after completed immunologic reaction → apoptosis of proliferating effector cells) - Also after exposure to injurious stimuli (eg, radiation, toxins, hypoxia) |
|
|
What happens during the Intrinsic Pathway of Apoptosis?
|
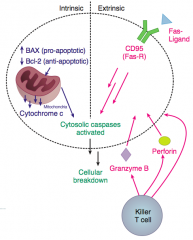
- Changes in proportions of anti-apoptotic factors (Bcl-2) and pro-apoptotic factors (BAX and BAK)
- Leads to ↑ mitochondrial permeability - Cytochrome C released from mitochondria into cytoplasm - Cytochrome C activates cytosolic Caspases leading to cellular breakdown |
|
|
What are the anti-apoptotic factors involved in the intrinsic pathway of apoptosis?
|
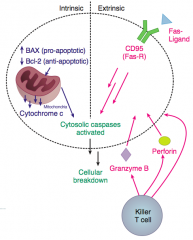
Bcl-2
|
|
|
What are the pro-apoptotic factors involved in the intrinsic pathway of apoptosis?
|
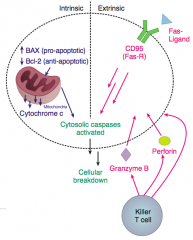
BAX and BAK
|
|
|
What is the action of Bcl-2?
|
Anti-apoptotic
- Prevents cytochrome C release from mitochondria by binding to and inhibiting Apaf-1 - Apaf-1 normally induces the activation of Caspases - If Bcl-2 is overexpressed (eg, follicular lymphoma), then Apaf-1 is overly inhibited, leading to ↓ Caspase activation and tumorigenesis |
|
|
What happens in the Fas pathway for apoptosis? What is its role?
|
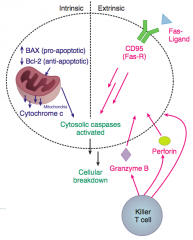
Extrinsic Pathway
- Fas-FasL interaction is necessary in thymic medullary negative selection - Fas is aka CD95 and when FasL binds, multiple Fas molecules coalesce, forming a binding site for a death domain containing adaptor protein FADD - FADD binds inactive Caspases, activating them, leading to cellular breakdown |
|
|
What are the implications of mutations in Fas?
|
Increases numbers of circulating self-reacting lymphocytes due to failure of clonal deletion (usually involved in thymic medullary negative selection)
*Basis of autoimmune disorders* |
|
|
What is the molecular basis of autoimmune disorders?
|
Defective Fas-FasL interaction (important for thymic medullary negative selection)
|
|
|
What causes necrosis?
|
Enzymatic degradation and protein denaturation of a cell resulting from exogenous injury
|
|
|
What happens during necrosis?
|
- Intracellular components leak
- Inflammation |
|
|
How does apoptosis compare to necrosis?
|
Both result in death of cell(s), but necrosis causes inflammation
|
|
|
What are the types of necrosis?
|
- Coagulative
- Liquefactive - Caseous - Fatty - Fibrinoid - Gangrenous |
|
|
Which type of necrosis occurs in the heart, liver, and kidney? Characteristics?
|
Coagulative Necrosis
- Occurs in tissues supplied by end-arteries - Increased cytoplasmic binding of acidophilic dye - Proteins denature first, followed by enzymatic degradation |
|
|
Which type of necrosis occurs in the brain and bacterial abscesses? Characteristics?
|
Liquefactive Necrosis
- Occurs in CNS due to high fat content - In contract to coagulative necrosis, enzymatic degradation due to release of lysosomal enzymes occurs before proteins denature |
|
|
Which type of necrosis occurs with TB, systemic fungi, and Nocardia infections?
|
Caseous Necrosis
|
|
|
Which type of necrosis occurs in the pancreas and breast? Characteristics?
|
Fatty Necrosis
- Enzymatic: pancreatitis - saponification - Non-enzymatic: breast trauma - Calcium deposits appear dark blue on staining |
|
|
Which type of necrosis occurs in the vessels? Characteristics?
|
Fibrinoid Necrosis
- Vasculitides (eg, Henoch-Schönlein purpura, Churg-Strauss syndrome) - Malignant hypertension - Amorphous and pink on H&E |
|
|
Which type of necrosis occurs in the limbs and GI tract? Characteristics?
|
Gangrenous Necrosis
- Dry (ischemic coagulative) - Wet (infection) |
|
|
What are the characteristics of Coagulative Necrosis?
|
- Heart liver, kidney (occurs in tissues supplied by end-arteries
- Increased cytoplasmic binding of acidophilic dye - Proteins denature first, followed by enzymatic degradation |
|
|
What are the characteristics of Liquefactive Necrosis?
|
- Brain and bacterial abscesses
- Occurs in CNS due to high fat content - Enzymatic degradation by release of lysosomal enzymes occurs before proteins denature |
|
|
What are the causes of Caseous Necrosis?
|
- TB
- Systemic fungi - Nocardia |
|
|
What are the characteristics of Fatty Necrosis?
|
- Enzymatic (pancreatitis - saponification)
- Non-enzymatic (eg, breast tissue) - Calcium deposits appear dark blue on staining |
|
|
What are the characteristics of Fibrinoid Necrosis?
|
- Vasculitides (eg, Henoch-Schönlein purpura, Churg-Strauss syndrome)
- Malignant hypertension - Amoprhous and pink on H&E |
|
|
What are the characteristics of Gangrenous Necrosis?
|
- Common in limbs and GI tract
- Dry (ischemic coagulative) - Wet (infection) |
|
|
What are the characteristics of reversible cell injury?
|
Reversible with O2
- ATP depletion - Cellular / mitochondrial swelling (↓ ATP → ↓ activity of Na+/K+ pumps) - Nuclear chromatin clumping - ↓ Glycogen - Fatty change - Ribosomal / polysomal detachment (↓ protein synthesis) - Membrane blebbing |
|
|
What happens to the size of cells / mitochondria during reversible cell injury?
|
Cellular / mitochondrial swelling (↓ ATP → ↓ activity of Na+/K+ pumps)
|
|
|
What are the characteristics of irreversible cell injury?
|
- Nuclear pyknosis, karyorrhexis, karyolysis
- Plasma membrane damage (degradation of membrane phospholipid) - Lysosomal rupture - Mitochondrial permeability / vacuolization; phosopholipid-containing amorphous densities within mitochondria (swelling alone is reversible) |
|
|
Which organs are susceptible to hypoxia / ischemia and infarction?
|
- Brain
- Heart - Kidney - Liver - Colon |
|
|
Which areas of the brain are most susceptible to hypoxia / ischemia and infarction?
|
Boundary / watershed areas
- ACA / MCA - MCA / PCA These areas receive dual blood supply from most distal branches of 2 arteries, which protects these areas from single-vessel focal blockage; however they are susceptible to ischemia from systemic hyperperfusion |
|
|
Specifically, which cells are most susceptible to the effects of hypoxia in the brain?
|
Hypoxic ischemic encephalopathy (HIE) affects:
- Pyramidal cells of hippocampus - Purkinje cells of cerebellum |
|
|
Which area of the heart is most susceptible to hypoxia / ischemia and infarction?
|
Subendocardium (LV)
|
|
|
Which areas of the kidney are most susceptible to hypoxia / ischemia and infarction?
|
- Straight segment of proximal tubule (medulla)
- Thick ascending limb (medulla) |
|
|
Which area of the liver is most susceptible to hypoxia / ischemia and infarction?
|
Area round central vein (zone III)
|
|
|
Which areas of the colon are most susceptible to hypoxia / ischemia and infarction?
|
- Splenic flexure
- Rectum These areas receive dual blood supply from most distal branches of 2 arteries, which protects these areas from single-vessel focal blockage; however they are susceptible to ischemia from systemic hyperperfusion |
|
|
What causes reperfusion injury?
|
Free radicals
|
|
|
What are the types of infarcts?
|
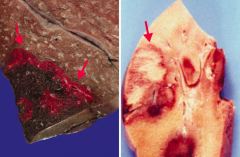
Red (left) vs Pale (right)
|
|
|
What are the characteristics of "red infarcts"?
|
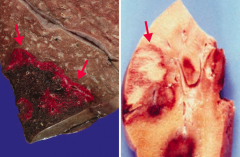
- Red (hemorrhagic) infarcts occur in loose tissues with multiple blood supplies (left)
- Liver, lungs, intestine - Red = Reperfusion (injury is due to damage by free radicals) |
|
|
What are the characteristics of "pale infarcts"?
|
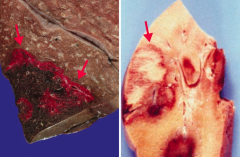
- Pale infarcts occur in solid tissues with a single blood supply (right)
- Heart, kidney, spleen |
|
|
What is the first sign of shock?
|
Tachycardia
|
|
|
What is shock in the setting of DIC secondary to?
|
Secondary to trauma - likely due to sepsis
|
|
|
What are the types of shock?
|
- Distributive
- Hypovolemic / cardiogenic |
|
|
What type of shock occurs due to high-output failure?
|
Distributive Shock
- ↓ TPR - ↑ CO - ↑ Venous Return |
|
|
What type of shock occurs due to low-output failure?
|
Hypovolemic / Cardiogenic Shock
- ↑ TPR - ↓ CO - ↓ Venous Return |
|
|
What is the relative pulmonary capillary wedge pressure (PCWP) in the different types of shock?
|
- Distributive: ↓
- Hypovolemic: ↓ - Cardiogenic: ↑ |
|
|
What type of shock is associated with vasodilation? Symptoms associated with this?
|
Distributive - warm, dry skin
|
|
|
What type of shock is associated with vasoconstriction? Symptoms associated with this?
|
Hypovolemic & Cardiogenic
- Cold, clammy patient |
|
|
How do IV fluids affect distributive shock?
|
Failure to ↑ BP with IV fluids
|
|
|
How do IV fluids affect hypovolemic / cardiogenic shock?
|
Blood pressure restored with IV fluids
|
|
|
Which type of shock can not restore the BP with IV fluids?
|
Distributive shock
|
|
|
Which type of shock has a BP that can be restored by IV fluids?
|
Hypovolemic / Cardiogenic shock
|
|
|
What happens with atrophy?
|
Reduction in the size and / or number of cells
|
|
|
What can cause atrophy?
|
- ↓ Endogenous hormones (eg, post menopausal ovaries)
- ↑ Exogenous hormones (eg, factitious thyrotoxicosis, steroid use) - ↓ Innervation (eg, motor neuron damage) - ↓ Blood flow / nutrients - ↓ Metabolic demand (eg, prolonged hospitalization, paralysis) - ↑ Pressure (eg, nephrolithiasis) - Occlusion of secretory ducts (eg, cystic fibrosis) |
|
|
How can the levels of hormones lead to atrophy? Examples?
|
- ↓ Endogenous hormones (eg, post-menopausal ovaries)
- ↑ Exogenous hormones (eg, factitious thyrotoxicosis, steroid use) |
|
|
How can a change in innervation lead to atrophy? Example?
|
↓ Innervation (eg, motor neuron damage)
|
|
|
How can a change in blood flow / nutrients lead to atrophy?
|
↓ Blood flow / nutrients
|
|
|
How can a change in metabolic demand lead to atrophy? Examples?
|
↓ Metabolic demand (eg, prolonged hospitalization or paralysis)
|
|
|
How can a change in pressure lead to atrophy? Example?
|
↑ Pressure (eg, nephrolithiasis)
|
|
|
How can a change in secretory ducts lead to atrophy? Example?
|
Occlusion of secretory ducts (eg, cystic fibrosis)
|
|
|
What are the signs of inflammation?
|
- Rubor (redness)
- Dolor (pain) - Calor (heat) - Tumor (swelling) - Functio laesa (loss of function) |
|
|
What happens to the vascular component with inflammation?
|
↑ Vascular permeability, vasodilation, and endothelial injury
|
|
|
What happens to the cellular component with inflammation?
|
Neutrophils extravasate from circulation to injured tissue to participate in inflammation through phagocytosis, degranulation, and inflammatory mediator release
|
|
|
What are the effects of neutrophils in inflammation?
|
- Phagocytosis
- Degranulation - Inflammatory mediator release |
|
|
What mediates acute cellular inflammation?
|
- Neutrophils
- Eosinophils - Antibodies |
|
|
What is the time course of acute inflammation?
|
Rapid onset (seconds to minutes) - lasts minutes to days
|
|
|
What are the potential outcomes of acute inflammation?
|
- Complete resolution
- Abscess formation - Progression to chronic inflammation |
|
|
What mediates chronic cellular inflammation?
|
- Mononuclear cells
- Fibroblasts |
|
|
What characterizes chronic cellular inflammation?
|
- Persistent destruction and repair
- Associated with blood vessel proliferation and fibrosis |
|
|
What is a granuloma made of?
|
Nodular collection of epithelioid macrophages and giant cells
|
|
|
What are the potential outcomes of chronic inflammation?
|
- Scarring
- Amyloidosis |
|
|
What is the term for the process involving the cell body following axonal injury?
|
Chromatolysis
|
|
|
What is Chromatolysis?
|
- Process involving the cell body following axonal injury
- Changes reflect ↑ protein synthesis in effort to repair damaged axon |
|
|
What are the characteristics of Chromatolysis?
|
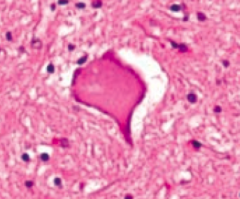
- Round cellular swelling
- Displacement of nucleus to periphery - Dispersion of Nissl substance throughout cytoplasm |
|
|
What are the types of "calcification"?
|
- Dystrophic calcification
- Metastatic calcification |
|
|
What is the term for calcium deposition in tissues secondary to necrosis?
|
Dystrophic Calcification
|
|
|
Where does dystrophic calcification occur?
|
Tissues with necrosis
- Tends to be localized (eg, on heart valves) |
|
|
What is dystrophic calcification associated with?
|
- TB (lungs and pericardium)
- Liquefactive necrosis of chronic abscesses - Fat necrosis - Infarcts and thrombi - Schistosomiasis - Mönckeberg arteriolosclerosis - Congenital CMV + Toxoplasmosis - Psamomma bodies |
|
|
What is the relationship of dystrophic calcification to the level of calcium in the serum?
|
Not directly associated with hypercalcemia (ie, patients are usually normocalcemic)
|
|
|
Where does metastatic calcification occur?
|
Widespread (ie, diffuse and metastatic) deposition in normal tissue
- Predominantly in interstitial tissues of kidney, lungs, and gastric mucosa |
|
|
What causes metastatic calcification?
|
Deposits in normal tissue secondary to:
- Hypercalcemia (eg, primary hyperthyroidism, sarcoidosis, hypervitaminosis D) - High calcium-phosphate product (eg, chronic renal failure + secondary hyperthyroidism, long-term dialysis, calciphylaxis, warfarin) |
|
|
What can cause hypercalcemia leading to metastatic calcification?
|
- Primary hyperparathyroidism
- Sarcoidosis - Hypervitaminosis D |
|
|
What can cause high calcium-phosphate product leading to metastatic calcification?
|
- Chronic renal failure + secondary hyperparathyroidism
- Long-term dialysis - Calciphylaxis - Warfarin |
|
|
What are the characteristics of the kidney, lungs, and gastric mucosa that favors metastatic calcification? How promotes deposition?
|
- These tissues lose acid quickly
- ↑ pH favors deposition |
|
|
What are the levels of calcium in patients with metastatic calcification?
|
Patients are usually not normocalcemic
|
|
|
What is the most common location for leukocyte extravasation?
|
Post-capillary venules (sites of tissue injury and inflammation)
|
|
|
What are the four steps of leukocyte extravasation?
|
1. Margination and rolling
2. Tight-binding 3. Diapedesis 4. Migration |
|
|
What mediates the first step of leukocyte extravasation?
|
Margination and Rolling:
- E-selectin binds Sialyl-Lewis - P-selectin binds Sialyl-Lewis - GlyCAM-1, CD34 bind L-selectin |
|
|
What mediates the second step of leukocyte extravasation, after margination and rolling?
|
Tight-Binding
- ICAM-1 (CD54) binds CD11/18 integrins (LFA-1, Mac-1) - VCAM-1 (CD106) binds VLA-4 integrin |
|
|
What mediates the third step of leukocyte extravasation, after tight-binding?
|
Diapedesis - leukocyte travels between endothelial cells and exits blood vessel
- PECAM-1 (CD31) binds PECAM-1 (CD31) |
|
|
What mediates the fourth step of leukocyte extravasation, after diapedesis?
|
Migration - leukocyte travels through interstitium to site of injury or infection guided by chemotactic signals
|
|
|
What chemotactic products are release in response to bacteria to stimulate leukocyte migration?
|
- C5a
- IL-8 - LTB4 - Kallikrein - Platelet-actvating factor |
|
|
What mediates margination and rolling in leukocyte extravasation?
|
Vasculature / stroma:
- E-selectin - P-selectin - GlyCAM-1, CD34 Leukocyte: - Sialyl-Lewis - L-selectin |
|
|
What mediates tight binding in leukocyte extravasation?
|
Vasculature / stroma:
- ICAM-1 (CD54) - VCAM-1 (CD106) Leukocyte: - CD11/18 integrins (LFA-1, Mac-1) - VLA-4 integrin |
|
|
What is the term for when a leukocyte travels between endothelial cells to exit a blood vessel?
|
Diapedesis
|
|
|
What mediates diapedesis in leukocyte extravasation?
|
PECAM-1 (CD31) on both vasculature/stroma and leukocytes
|
|
|
How do free radicals damage cells?
|
- Lipid peroxidation
- Protein modification - DNA breakage |
|
|
What can initiate free radical damage?
|
- Radiation exposure (eg, cancer therapy)
- Metabolism of drugs (phase I) - Redox reactions - Nitric oxide - Transition metals - Leukocyte oxidative burst |
|
|
What enzymes can eliminate free radicals?
|
- Catalase
- Superoxide dismutase - Glutathione peroxidase |
|
|
Besides enzymes, what else can eliminate free radicals?
|
- Spontaneous decay
- Antioxidants (eg, vitamins A, C, and E) |
|
|
What pathologies are caused by free radical injury?
|
- Retinopathy of prematurity
- Bronchopulmonary dysplasia - Carbon tetrachloride, leading to liver necrosis (fatty change) - Acetaminophen overdose (fulminant hepatitis, renal papillary necrosis) - Iron overload (hemochromatosis) - Reperfusion injury (eg, superoxide), especially after thrombolytic therapy |
|
|
What free radical damage is associated with prematurity?
|
Retinopathy
|
|
|
How can the lungs be affected by free radical damage?
|
Bronchopulmonary Dysplasia
|
|
|
What are the effects of carbon tetrachloride?
|
Free radical damage → liver necrosis (fatty change)
|
|
|
What are the free radical effects of acetaminophen overdose?
|
Overdose → fulminant hepatitis and renal papillary necrosis
|
|
|
What mediates reperfusion injury?
|
Superoxide, especially after thrombolytic therapy
|
|
|
What is the most common pulmonary complication after exposure to fire?
|
Inhalation Injury:
- Inhalation of products of combustion (eg, carbon particles, toxic fumes) → chemical tracheobronchitis, edema, and pneumonia |
|
|
How long does it take for wound healing to get a majority of the tensile strength back to the tissue? What percentage of tensile strength?
|
Takes ~3 months following wound formation to get 70-80% of the tensile strength back (little additional strength will be regained after that)
|
|
|
What are the pathologic types of scars?
|
- Hypertrophic scars
- Keloid scars |
|
|
Which type of scar has greater collagen synthesis?
|
- Keloid Scars: ↑↑↑ collagen synthesis
- Hypertrophic Scars: ↑ collagen synthesis |
|
|
What is the arrangement of collagen in hypertrophic vs keloid scars?
|
- Hypertrophic: parallel collagen
- Keloid: disorganized collagen |
|
|
What is the extent of a hypertrophic vs keloid scars?
|
- Hypertrophic: confined to borders of original wound
- Keloid: extends beyond the borders of the original wound |
|
|
Do hypertrophic scars tend to recur following resection? vs keloid scars?
|
- Hypertrophic scars infrequently recur following resection
- Keloid scars frequently recur following resection |
|
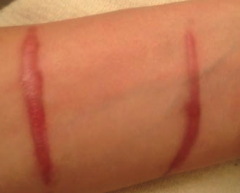
What types of scars are these? Characteristics?
|
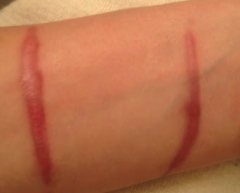
Keloid Scars
- ↑ collagen synthesis - Parallel collagen arrangement - Scar is confined to borders of original wound - Infrequently recurs following resection |
|
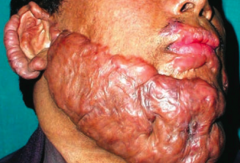
What types of scars are these? Characteristics?
|
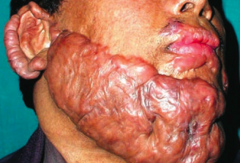
Keloid Scars
- ↑↑↑ collagen synthesis - Disorganized collagen arrangement - Scar extends beyond the borders of the original wound - Frequently recurs following resection |
|
|
Who is at higher risk for keloid scars?
|
African-Americans
|
|
|
What are the tissue mediators of wound healing?
|
- PDGF
- FGF - EGF - TGF-β - Metalloproteinases |
|
|
What is the source of PDGF? Function?
|
PDGF is secreted by activated platelets and macrophages
- Induces vascular remodeling and smooth muscle cell migration - Stimulates fibroblast growth for collagen synthesis |
|
|
What is the function of FGF?
|
Stimulates all aspects of angiogenesis
|
|
|
What is the function of EGF?
|
Stimulates cell growth via tyrosine kinases (eg, EGFR as expressed by ERBB2)
|
|
|
What is the function of TGF-β?
|
- Angiogenesis
- Fibrosis - Cell cycle arrest |
|
|
What is the function of metalloproteinases?
|
Tissue remodeling
|
|
|
What factor induces vascular remodeling and smooth muscle cell migration?
|
PDGF (from activated platelets and macrophages)
|
|
|
What factor stimulates fibroblast growth for collagen synthesis?
|
PDGF (from activated platelets and macrophages)
|
|
|
What factor stimulates all aspects of angiogenesis?
|
FGF
|
|
|
What factor stimulates cell growth via tyrosine kinases?
|
EGF - via EGFR, as expressed by ERBB2
|
|
|
What enzyme is involved in tissue remodeling?
|
Metalloproteinases
|
|
|
What are the phases of wound healing?
|
1. Inflammatory (immediate)
2. Proliferative (2-3 days after wound) 3. Remodeling (1 week after wound) |
|
|
What is the immediate phase of wound healing? Mediators?
|
Inflammatory Phase
- Mediated by platelets, neutrophils, macrophages |
|
|
What is the phase of wound healing that occurs 2-3 days after a wound? Mediators?
|
Proliferative Phase
- Fibroblasts - Myofibroblasts - Endothelial cells - Keratinocytes - Macrophages |
|
|
What is the phase of wound healing that occurs 1 week after a wound? Mediators?
|
Remodeling Phase
- Fibroblasts |
|
|
What are the characteristics of the inflammatory phase of wound healing?
|
Immediately after wound:
- Clot formation - ↑ Vessel permeability and neutrophil migration into tissues - Macrophages clear debris 2 days later |
|
|
What are the characteristics of the proliferative phase of wound healing?
|
2-3 days after wound
- Deposition of granulation tissue and collagen - Angiogenesis - Epithelial cell proliferation - Dissolution of clot - Wound contraction (mediated by myofibroblasts) |
|
|
What are the characteristics of the remodeling phase of wound healing?
|
1 week after wound
- Type III collagen replaced by type I collagen - Increased tensile strength of tissue |
|
|
What bugs/pathologies can cause granulomas?
|
- Bartonella henselae (cat scratch)
- Berylliosis - Churg-Strauss syndrome - Crohn disease - Francisella tularensis - Fungal infections (eg, histoplasmosis, blastomycosis) - Granulomatosis with polyangiitis (Wegener) - Listeria monocytogenes (granulomatosis infantisepticemia) - M. leprae (leprosy; Hansen disease) - M. tuberculosis - Treponema pallidum (tertiary syphilis) - Sarcoidosis - Schistosomiasis |
|
|
What mediates the formation of a granuloma?
|
- Th1 cells secrete γ-interferon, activating macrophages
- Macrophages release TNF-α, which induces and maintains granuloma formation |
|
|
What can the side effects of anti-TNF drugs be?
|
Cause sequestering granulomas to breakdown, leading to disseminated disease
(TNF-α maintains granuloma formation) |
|
|
Which mediator activates macrophages? Source?
|
γ-Interferon from Th1 cells
|
|
|
What mediator from macrophages induces and maintains granuloma formation?
|
TNF-α
|
|
|
What do you need to check for before starting anti-TNF therapy? Why?
|
Latent Tuberculosis
- Anti-TNF drugs can cause sequestering granulomas to breakdown, leading to disseminated disease |
|
|
Which is thicker/thinner: exudate or transudate?
|
- Exudate (thick)
- Transudate (thin) |
|
|
What are the contents of an exudate?
|
- Cellular
- Protein rich |
|
|
What are the contents of an transudate?
|
- Hypocellular
- Protein-poor |
|
|
What is the relative specific gravity in an exudate vs transudate?
|
- Exudate: >1.020 (thick)
- Transudate: <1.012 (thin) |
|
|
What can cause an exudate?
|
- Lymphatic obstruction
- Inflammation / infection - Malignancy |
|
|
What can cause a transudate?
|
- ↑ Hydrostatic pressure (eg, CHF)
- ↓ Oncotic pressure (eg, cirrhosis) - Na+ retention |
|
|
What is the Erythrocyte Sedimentation Rate a reflection of?
|
- Products of inflammation (eg, fibrinogen) coat RBCs and cause aggregation
- When aggregated, RBCs fall at a faster rate within the test tube |
|
|
What can cause increased ESR?
|
- Most anemias
- Infections - Inflammation (eg, temporal arteritis) - Cancer (eg, multiple myeloma) - Pregnancy - Autoimmune disorders (eg, SLE) |
|
|
What can cause decreased ESR?
|
- Sickle cell (altered shape)
- Polycythemia (↑ RBCs "dilute" aggregation factors) - CHF (unknown) |
|
|
What is one of the leading causes of fatality from toxicologic agents in children?
|
Iron poisoning
|
|
|
What is the mechanism of iron poisoning?
|
Cell death due to peroxidation of membrane lipids
|
|
|
What are the acute symptoms of iron poisoning?
|
- Nausea
- Vomiting - Gastric bleeding - Lethargy |
|
|
What are the chronic symptoms of iron poisoning?
|
- Metabolic acidosis
- Scarring leading to GI obstruction |
|
|
How do you treat a patient with iron poisoning?
|
Chelation:
- IV deferoxamine - Oral deferasirox Dialysis |
|
|
What is the term for the abnormal aggregation of proteins (or their fragments) into β-pleated sheet structures? Implications?
|
Amyloidosis → damage and apoptosis
|
|
|
What are the common types of Amyloidsosi?
|
- AL (primary)
- AA (secondary) - Dialysis-related - Heritable - Age-related (senile) systemic - Organ specific |
|
|
What causes AL (primary) amyloidosis? Associated with?
|
- Deposition of proteins from Ig Light chains
- Can occur as a plasma cell disorder or associated with multiple myeloma |
|
|
What are the extent of the effects of AL (primary) amyloidosis?
|
Often affects multiple organ systems, including:
- Renal (nephrotic syndrome) - Cardiac (restrictive cardiomyopathy, arrhythmia) - Hematologic (easy bruising) - GI (hepatomegaly) - Neurologic (neuropathy) |
|
|
What causes AA (secondary) amyloidosis? Associated with?
|
Seen with chronic conditions:
- Rheumatoid arthritis - IBD - Spondyloarthropathy - Protracted infection Often multisystem |
|
|
What is AA (secondary) amyloidosis composed of?
|
Fibrils composed of serum Amyloid A
|
|
|
What are the extent of the effects of AA (secondary) amyloidosis?
|
Often multisystem involvement like AL amyloidosis
|
|
|
How does dialysis relate to amyloidosis?
|
Dialysis-Related Amyloidosis:
- Fibrils composed of β2-microglobulin in patients with ESRD and/or on long-term dialysis |
|
|
How might dialysis-related amyloidosis present?
|
Carpal tunnel syndrome
|
|
|
What is the amyloidosis in patients on dialysis composed of?
|
β2-microglobulin
|
|
|
What are the characteristics of heritable amyloidosis? Cause?
|
Heterogenous group of disorders
- Example is ATTR neurologic / cardiac amyloidosis due to transthyretin (TTR or prealbumin) gene mutation |
|
|
What causes age-related (senile) systemic amyloidosis?
|
Deposition of normal (wild-type) transthyretin (TTR) in myocardium and other sites
|
|
|
How does the rate of cardiac dysfunction compare in age-related amyloidosis and AL amyloidosis?
|
Age-related amyloidosis has a slower progression of cardiac dysfunction relative to AL amyloidosis
|
|
|
What is the most important form of organ-specific amyloidosis? Cause?
|
Alzheimer Disease
- Deposition of amyloid-β protein cleaved from amyloid precursor protein (APP) |
|
|
What type of amyloidosis is associated with T2DM? Cause?
|
Islet Amyloid Polypeptide (IAPP) - type of organ-specific amyloidosis
- Commonly seen in T2DM - Caused by deposition of amylin in pancreatic islets |
|
|
What type of disease is caused by deposition of amylin in the pancreatic islets?
|
Islet Amyloid Polypeptide (IAPP) - organ-specific amyloidosis commonly seen in patients with T2DM
|
|
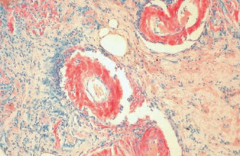
What does this image show?
|
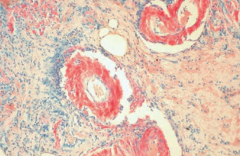
Amyloidosis:
- Congo red stain shows amyloid deposits within vessel walls |
|
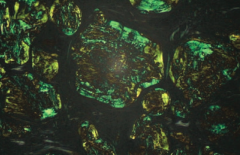
What does this image show?
|
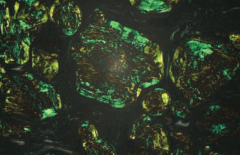
Amyloidosis
- Congo red stain shows apple green birefringence under polarized light |
|
|
What is the name of the yellow-brown "wear and tear" pigment associated with normal aging?
|
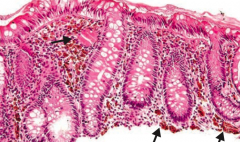
Lipofuscin
- Macrophages with granular yellow-brown pigment |
|
|
What is the cause of Lipofuscin deposition in macrophages?
|
Formed by oxidation and polymerization of auto-phagocytosed organellar membranes
|
|
|
Where will you find Lipofuscin in an autopsy of an elderly person?
|
- Heart
- Liver - Kidney - Eye - Other organs |

