![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
66 Cards in this Set
- Front
- Back
|
What are the acute manifestations of diabetes mellitus?
|
- Polydipsia
- Polyuria - Polyphagia - Weight loss - DKA (diabetic ketoacidosis) (type 1) - Hyperosmolar coma (type 2) |
|
|
What can, although rarely, cause Diabetes Mellitus?
|
Unopposed secretion of GH and Epinephrine
|
|
|
What are the immediate consequences of an insulin deficiency (and glucagon excess)?
|
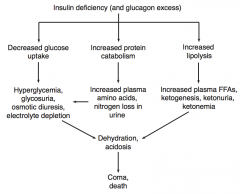
- Decreased glucose uptake
- Increased protein catabolism - Increased lipolysis |
|
|
What is the effect of decreased glucose uptake (due to insulin deficiency / glucagon excess)?
|
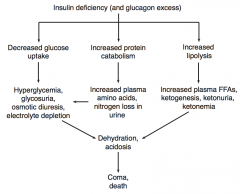
- Hyperglycemia
- Glycosuria (excess of sugar in the urine) - Osmotic diuresis - Electrolyte depletion |
|
|
What is the effect of increased protein catabolism (due to insulin deficiency / glucagon excess)?
|
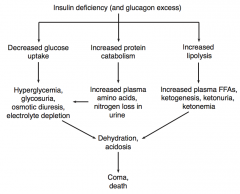
- Increased plasma amino acids
- Nitrogen loss in urine |
|
|
What is the effect of increased lipolysis (due to insulin deficiency / glucagon excess)?
|
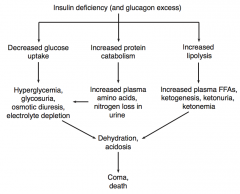
- Increased plasma FFAs
- Ketogenesis - Ketonuria - Ketonemia |
|
|
What is the combined effect of decreased glucose uptake, increased protein catabolism, and increased lipolysis (due to insulin deficiency / glucagon excess)?
|
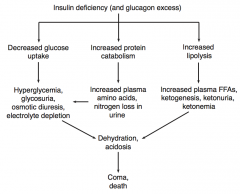
Dehydration and acidosis, which can cause coma or death
|
|
|
What are the types of damage due to chronic diabetes?
|
- Non-enzymatic glycosylation: small vessel and large vessel disease
- Osmotic damage: neuropathy, cataracts |
|
|
What are the manifestations of non-enzymatic glycosylation on small vessels in patients with chronic diabetes?
|
Diffuse thickening of basement membrane of small vessels leads to:
- Retinopathy - Glaucoma - Nephropathy |
|
|
How are the eyes of patients with chronic diabetes affected?
|
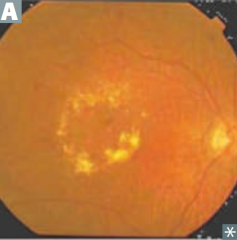
Small vessel disease due to diffuse thickening of basement membrane via non-enzymatic glycosylation:
- Retinopathy (picture): hemorrhage, exudates, microaneurysms, vessel proliferation - Glaucoma |
|
|
How are the kidneys of patients with chronic diabetes affected?
|
Small vessel disease (diffuse thickening of basement membrane) via non-enzymatic glycosylation:
- Nephropathy: nodular sclerosis, progressive proteinuria, chronic renal failure, arteriolosclerosis leading to HTN, Kimmelstiel-Wilson nodules) |
|
|
What are the manifestations of retinopathy in chronic diabetes mellitus?
|
- Hemorrhage
- Exudates - Microaneurysms - Vessel proliferation |
|
|
What are the manifestations of nephropathy in chronic diabetes mellitus?
|
- Nodular sclerosis
- Progressive proteinuria - Chronic renal failure - Arteriolosclerosis leading to HTN - Kimmelstiel-Wilson nodules |
|
|
What are the manifestations of non-enzymatic glycosylation on large vessels in patients with chronic diabetes?
|
- Large vessel atherosclerosis → cerebrovascular disease
- CAD → MI (most common cause of death) - Peripheral vascular occlusive disease - Gangrene → limb loss |
|
|
What is the most common cause of death in patients with Diabetes Mellitus?
|
Myocardial Infarction
|
|
|
What causes osmotic damage in patients with chronic diabetes?
|
Sorbitol accumulates in organs with aldose reductase and ↓ or absent sorbitol dehydrogenase
|
|
|
What are the manifestations of osmotic damage in patients with chronic diabetes?
|
- Neuropathy: motor, sensory, and autonomic degeneration
- Cataracts |
|
|
What tests can be used to assess a patient's diabetes mellitus?
|
- Fasting serum glucose
- Oral glucose tolerance test - HbA1c (reflects average blood glucose over prior 3 months) |
|
|
What is the primary defect in T1DM vs T2DM?
|
- T1DM: auto-immune destruction of β cells
- T2DM: ↑ resistance to insulin, progressive pancreatic β-cell failure |
|
|
Is insulin necessary in treatment of T1DM and T2DM?
|
- T1DM: always
- T2DM: sometimes |
|
|
What is the typical age of onset for patients with T1DM vs T2DM?
|
- T1DM: <30 years
- T2DM: >40 years *Exceptions commonly occur |
|
|
What is the association with obesity for T1DM vs T2DM?
|
- T1DM: none
- T2DM: associated |
|
|
Is there a genetic predisposition for T1DM vs T2DM?
|
- T1DM: relatively weak (50% concordance in identical twins), polygenic
- T2DM: relatively strong (90% concordance in identical twins), polygenic |
|
|
What is the association with HLA system for T1DM vs T2DM?
|
- T1DM: associated with HLA-DR3 and -DR4
- T2DM: no association |
|
|
What is the relative glucose intolerance in T1DM vs T2DM?
|
- T1DM: severe
- T2DM: mild to moderate |
|
|
What is the relative insulin sensitivity in T1DM vs T2DM?
|
- T1DM: high
- T2DM: low |
|
|
How common is ketoacidosis in T1DM vs T2DM?
|
- T1DM: common
- T2DM: rare |
|
|
How many β-cells are there in the islets relatively in T1DM vs T2DM?
|
- T1DM: ↓ β-cell numbers
- T2DM: variable (with amyloid deposits) |
|
|
What is the relative serum insulin level in T1DM vs T2DM?
|
- T1DM: ↓
- T2DM: Variable |
|
|
Are the classic symptoms of polyuria, polydipsia, polyphagia, and weight loss seen in T1DM vs T2DM?
|
- T1DM: common
- T2DM: sometimes |
|
|
What is the histologic appearance of the Islets of Langerhans in T1DM vs T2DM?
|
- T1DM: islet leukocytic infiltrate (auto-immune process)
- T2DM: islet amyloid polypeptide (IAPP) deposits |
|
|
What is one of the most important complications of diabetes (usually type 1)?
|
Diabetic Ketoacidosis
|
|
|
What causes diabetic ketoacidosis?
|
- Complication of diabetes (usually type 1)
- Usually due to ↑ insulin requirements from ↑ stress (eg, infection) - Excess fat breakdown and ↑ ketogenesis from ↑ FFAs, when are converted into ketone bodies |
|
|
What are the types of ketone bodies? Which is more common in diabetic ketoacidosis?
|
β-Hydroxybutyrate > Acetoacetate
|
|
|
What are the signs / symptoms of diabetic ketoacidosis?
|
- Kussmaul respirations (rapid / deep breathing)
- Nausea / vomiting - Abdominal pain - Psychosis / delirium - Dehydration - Fruity breath odor (due to exhaled acetone) |
|
|
What are Kussmaul respirations? Sign of?
|
- Rapid / deep breathing
- Sign of diabetic ketoacidosis |
|
|
What causes the fruity breath odor in diabetics?
|
Diabetic ketoacidosis → exhaled acetone
|
|
|
What are the lab findings associated with diabetic ketoacidosis?
|
- Hyperglycemia
- ↑ H+ and ↓ HCO3- (anion gap metabolic acidosis) - ↑ Blood ketone levels - Leukocytosis - Hyperkalemia, but depleted intracellular K+ d/t transcellular shift from ↓ insulin |
|
|
What kind of acid/base disturbance occurs with diabetic ketoacidosis?
|
Anion gap metabolic acidosis
- ↑ H+ - ↓ HCO3- |
|
|
How are WBCs affected in diabetic ketoacidosis?
|
Leukocytosis
|
|
|
How is K+ balance affected in diabetic ketoacidosis?
|
- Hyperkalemia
- Depleted intracellular K+ because of transcellular shift from ↓ insulin |
|
|
What are the potenial complications of diabetic ketoacidosis?
|
- Life-threatening mucormycosis (usually caused by Rhizopus infection)
- Cerebral edema - Cardiac arrhythmias - Heart failure |
|
|
How do you treat diabetic ketoacidosis?
|
- IV fluids
- IV insulin - K+ (to replete intracellular stores) - Glucose if necessary to prevent hypoglycemia |
|
|
What is the source of an insulinoma? What is its effect?
|
- Tumor of β cells of pancreas
- Over-produces insulin → hypoglycemia |
|
|
What are the common symptoms with an insulinoma?
|
Whipple triad of episodic CNS symptoms:
- Lethargy - Syncope - Diplopia |
|
|
What lab values are associated with insulinoma?
|
- ↓ Blood glucose
- ↑ C-peptide (vs exogenenous insulin use which would cause similar findings but have low/normal C-peptide) |
|
|
How do you treat an insulinoma?
|
Surgical resection of tumor in pancreas
|
|
|
What is the most common malignancy in the small intestine?
|
Carcinoid Syndrome
|
|
|
What causes Carcinoid Syndrome?
|
Rare syndrome caused by carcinoid tumors (neuroendocrine cells), especially metastatic small bowel tumors, which secrete high levels of serotonin (5-HT)
|
|
|
What is necessary for a carcinoid tumor of the small intestine to cause Carcinoid Syndrome?
|
- Tumor secretes high levels of serotonin (5-HT)
- Tumor must not be restricted to the GI tract because 5-HT undergoes first pass metabolism in the liver |
|
|
What are the symptoms of Carcinoid Syndrome?
|
- Recurrent diarrhea
- Cutaneous flushing - Asthmatic wheezing - Right sided valvular disease |
|
|
What are the lab changes associated with Carcinoid Syndrome?
|
- ↑ 5-hydroxyindoleacetic acid (5-HIAA) in urine
- Niacin deficiency (pellagra - diarrhea, dementia, dermatitis) |
|
|
How do you treat Carcinoid Syndrome?
|
- Resection of carcinoid tumor
- Somatostatin analog (eg, octreotide) |
|
|
What is the rule of 1/3 for Carcinoid Syndrome?
|
- 1/3 metastasize
- 1/3 present with 2nd malignancy - 1/3 are multiple |
|
|
What causes Zollinger-Ellison Syndrome?
|
- Gastrin-secreting tumor of pancreas or duodenum
- Leads to acid hyper-secretion → recurrent ulcers in distal duodenum and jejunum |
|
|
Zollinger-Ellison Syndrome leads to ulcers where?
|
Distal duodenum and jejunum
|
|
|
What symptoms does a patient with Zollinger-Ellison Syndrome typically present with?
|
- Abdominal pain (peptic ulcer disease, distal ulcers)
- Diarrhea (malabsorption) |
|
|
What may Zollinger-Ellison Syndrome be associated with?
|
MEN 1
|
|
|
What are the types of Multiple Endocrine Neoplasias?
|
- MEN 1 (Wermer syndrome)
- MEN 2A (Sipple syndrome) - MEN 2B |
|
|
What kind of tumors are associated with MEN 1?
|
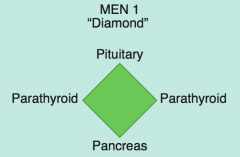
MEN 1 = 3 P's (diamond):
- Pituitary tumors (prolactin or GH) - Parathyroid tumors - Pancreatic endocrine tumors (Zollinger Ellison syndrome, insulinomas, VIPomas, glucagonomas-rare) |
|
|
Besides pituitary tumors, parathyroid tumors, and pancreatic endocrine tumors, what else is associated with MEN 1?
|
- Kidney stones
- Stomach ulcers |
|
|
What kind of tumors are associated with MEN 2A?
|
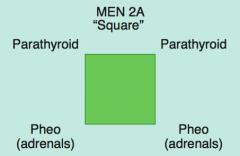
MEN 2A: 2 P's (square):
- Parathyroid (hyperplasia) - Pheochromocytoma (adrenals) - Medullary Thyroid Carcinoma (secretes calcitonin) |
|
|
Besides medullary thyroid carcinoma, pheochromocytoma, and parathyroid hyperplasia, what else is associated with MEN 2A?
|
Associated with RET gene mutations
|
|
|
What kind of tumors are associated with MEN 2B?
|
MEN 2B: 1 P (triangle):
- Pheochromocytoma (adrenals) - Oral / intestinal ganglioneuromatosis (mucosal neuromas) - Medullary Thyroid Carcinoma (secretes calcitonin) |
|
|
Besides medullary thyroid carcinoma, pheochromocytoma, and oral/intestinal ganglioneuromatosis (mucosal neuromas), what else is associated with MEN 2B?
|
Marfanoid habitus
- Resembling symptoms of Marfan Syndrome - Long limbs, arachnodactyly, and hyperlaxity - Arm span is greater than the height of the individual Associated with RET gene mutation |
|
|
How are MEN syndromes inherited? Other associated genetic changes?
|
- All are autosomal dominant (think "MEN are dominant" - or so they think)
- Associated with RET gene mutation in MEN 2A and 2B |

