![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
40 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What class of enzyme is chymotrypsin?
|
Protease
|
|
|
|
What are the four minimum compounds that need to be identified to understand the mechanism of a purified enzyme?
|
1. Substrates
2. Cofactors 3. Products 4. Regulators |
S, C, P, R
|
|
|
What are the five (5) aspects of the mechanism of a purified enzyme that need to be determined in order to understand the mechanism?
|
1. Sequence of enzyme-bound reaction intermediates' formation
2. Structure of intermediates and transitions states 3. Rates of interconversion between intermediates 4. Structural relationship of enzyme to each intermediate 5. Energy contributed by all reacting & interacting groups to intermediate comples and transition states |
Seq, Str(Int/Trns), Rate(IntCon), Str(Enzy-Int), E(each group)
|
|
|
What class of enzyme is hexokinase?
|
Transferase
Phosphotransferase (Polymerase & Kinase) -OH Acceptor |
|
|
|
What class of enzyme is enolase?
|
C-O Lyase
Hydrolyase (converts between enol forms) |
|
|
|
What class of enzyme is lysozyme?
|
Hydrolase (catalyzes hydrolysis)
Sugar Hydrolase Glycosidic Hydrolase |
|
|
|
What is chymotrypsinogen?
|
The inactive precursor of the enzyme chymotrypsin
|
|
|
|
Where is chymotrypsinogen produced?
|
The pancreas.
|
|
|
|
What is the first activation step from chymotrypsinogen to chymotrypsin?
|
Trypsin (changes to π-chymotrypsin)
|
|
|
|
What is the second activation step from chymotrypsinogen to chymotrypsin?
|
π-chymotrypsin autocleaves to yield α-chymptrypsin
|
|
|
|
What is a zymogen?
|
The inactive precursor to an enzyme. A biochemical change is necessary to activate the enzyme's functionality.
|
|
|
|
Why are zymogens important?
|
They prevent the functionality of a particular enzyme from being used in the wrong place or time. For example, digestive enzymes are inactive in the pancreas where they are formed, protecting the pancreas from digesting itself.
|
|
|
|
What does chymotrypsin catalyze?
|
The hydrolytic cleavage of peptide bonds.
|
|
|
|
Which amino acids will chymotrypsin cleave next to?
|
Aromatic amino acids: Trp, Phe, Tyr
|
|
|
|
What are the two phases of the chymotrypsin mechanism?
|
Acylation and deacylation
|
|
|
|
What else other than polypeptides will chymotrypsin hydrolyze? Is it faster or slower?
|
Small esters and amides. Slower than polypeptide hydrolysis because less binding energy is available from the substrate.
|
|
|
|
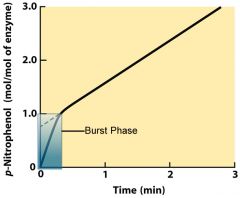
With respect to enzyme reaction kinetics, what is a burst phase?
|
It reveals a particular step in the enzyme mechanism which is faster than other steps, usually at the beginning, and only lasts a short time. Thus, the other slower steps bottleneck the reaction process, and the overall output of the enzyme slows down.
|
|
|
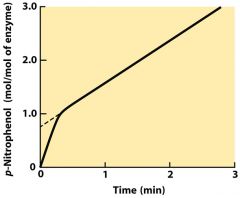
Identify the burst phase of the graph
|
|
|
|
|
At what pH does chymotrypsin have the optimal enzymatic activity (rate)?
|
pH = 8.0
|
|
|
|
What is a catalytic triad?
|
Three amino acids that work together to catalyze the reaction. They may not necessarily be close in the primary structure, but they are in the tertiary structure of the enzyme.
|
|
|
|
Which amino acids are involved in chymotrypsin's catalytic triad?
|
Ser, His, Asp (H-bonding network)
|
|
|
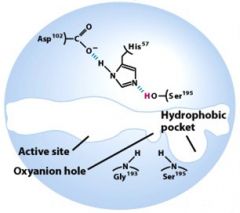
What enzyme (free) is this?
|
chymotrypsin
|
|
|
|
What is the purpose of the "oxyanion hold" in the mechanism for chymotrypsin?
|
It stabilizes the negative charge on the carbonyl oxygen of the peptide, adjacent to the cleavage site
|
|
|
|
In the chymotrypsin mechanism, after the nucleophilic attack of the serine's alkoxide on the carbonyl carbon, what is temporarily formed?
|
A tetrahedral intermediate. This is very short lived.
|
|
|
|
Why in the chymotrypsin mechanism is the tetrahedral intermediate so short lived?
|
The negative charge on the oxygen is very unstable, and the tetrahedral intermediate collapses, reforming the double bond
|
|
|
|
In the chymotrypsin mechanism, after the reformation of the carbonyl's double bond, why is the amino group (nitrogen) released from the carbon instead of the oxygen from the serine (which is the reverse of the original nucleophilic attack)?
|
Because the bond strength or disassociation energy of the C-N bond is weaker than the C-O bond. Plus, this allows the reaction to proceed forward (cleaving the peptide bond). Also, the nitrgoen gains a less electronegative atom in bonding with the hydrogen in place of the carbon.
|
|
|
|
In the chymotrypsin mechanism, how is the leaving amino acid group discharged?
|
The Histidine residue donates a proton to the Serine residue as its oxygen separates from the carbonyl group, unlinking it from the enzyme.
|
|
|
|
In the chymotrypsin mechanism, how is the deacylation phase initiated?
|
An incoming water molecule is deprotonated by the Histidine residue, causing the left over hydroxyide ion to nucleophilically attack the carbonyl. This again transfers electrons to the oxygen, which again temporarily holds this negative charge (fleetingly stabilized by Glycine and Serine residues at the active site).
|
|
|
|
In the chymotrypsin mechanism, in the deacylation phase, when the carbonyl is again reformed, why is the serine's oxygen released instead of the hydroxide that recently attached?
|
The serine's oxygen is hydrogen bonding with the hydrogen on the Histidine (taken from the water molecule), and is also bonded to the rest of the Serine. As the carbonyl is reformed, the extra electrons on the carbonyl carbon repulse the electrons on one of the oxygens. The serine's oxygen will be the most preferable choice since there's a hydrogen right next to it ready to replace the electrons being used on the carbon.
|
|
|
|
In the chymotrypsin mechanism, what is the last step of the deacylation phase?
|
It's the diffusion of the second product from the active site.
|
|
|
|
In the chymotrypsin mechanism, what is the rate limiting phase (not step)?
|
The deacylation phase.
|
|
|
|
What is the first step of the chymotrypsin mechanism?
|
Binding of the substrate (a polypeptide)
|
|
|
|
In the chymotrypsin mechanism, what happens after the binding of the substrate?
|
The enzyme slightly changes conformation, causing Asp and His to become slightly closer. This makes His more basic. It deprotonates the Serine, and the alkoxide from the Serine attacks the carbonyl group of the substrate.
|
|
|
|
Would chymotrypsin work better on a denatured or a regular protein?
|
It would work better on denatured proteins since it needs to get at the peptide bonds
|
|
|
|
Which enzyme kinetics parameter is most affected by changes to the transition state complementarity functional groups?
|
k.cat
|
|
|
|
What's one method of changing enzyme primary structure and sequence?
|
Site directed mutagenesis.
|
|
|
|
What is a transition state analog?
|
A stable structure that resembles a transition state. It allows us to guess more accurately at what a transition state might be in a mechanism.
|
|
|
|
What is a medical application of protease inhibitors?
|
Anti-viral medications for things like HIV or Hepatitis C.
|
|
|
|
What is an epitope?
|
The unique region of a biological structure that can be recognized by an antibody, B cell or T cell.
|
|
|
|
What reaction does hexokinase catalyze?
|
from β-D-Glucose to Glucose 6-phosphate (and back)
|
|

