![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
19 Cards in this Set
- Front
- Back
|
Properties of energy levels |
1.Energy level size increases as n increases
2. The energy of e-s in energy levels increases as n increases
3. The nember of electrons a level can hold: maximum population = 2n^2
4. Valence electrons Outer most energy level e-s Participate in chemical bonding (shared or transferred). .
|
|
|
For group elements: |
# Valence e-s = group number |
|
|
Quantum Theory aka Quantum Mechanical Model of an atom |

In this model, 4 quantum numbers give the location of electron. |
|
|
The principle Quantum Number (n) aka energy level |
Quantum number 1.
Sublevels: s,p,d,f Orbitals: s,p,d,f The orbital is where you have the highest probability of finding e- location. |
|
|
Heisenburg Uncertainty Principle |
It is impossible to know both the location and energy of an electron at the same time. The orbital is where you have the highest probability of finding e- location. |
|
|
Angular Momentum (l): |
The shape of the orbital.
S l=0 sphere P l=1 double sphere D l=2 double sphere with ring F l=3 flower |
|
|
Magnetic Quantum Number (Ml) |
Total number of orientations a particular orbital has in 3-D space S ml = 0 1 orientation P ml = -1, 0, +1 3 orientations D ml = -2, -1, 0, +1, +2 5 orientations F ml = -3, -2, -1, 0, +1, +2, +3 7 orientations |
|
|
Electron Spin (Ms) |
2 electrons in the same orbital must have opposite spin. Clockwise ^| +1/2 Counterclockwise |, -1/2 |
|
|
Electron order: |
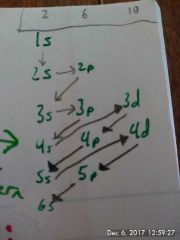
1s 2s 2p 3s 3p 4s 3d 4p 5s 4d 5p 6s Use periodic table, or |
|
|
Why do electrons fill up n=4 before n=3 is full? |
Energy levels get closer together as (n) quantum number increases. This causes sublevels to overlap. (Because of this bohr diagram only show accurate ve- for atomic numbers < 18) |
|
|
Degenerate Orbitals |
They let a higher energy level go in between. 3p 3d 4p 4d If an electron configuration ends in a degenerate orbital: completely fill or half fill with e-s from orbital below. |
|
|
Hunds rule |
Maximum number of e-s in degenerate orbital is the lowest energy configuration. |
|
|
Electron Configurations of ions |

|
|
|
Shorthand configs |

|
|
|
Orbital diagrams |
1. Spin 2. Box 3. Hunds rule, each orbital must have one electron before pairing. (All same spin) 4. Pauli's Exclusion Principle: paired electrons must have opposite spin. |
|
|
Orbital diagrams 2 |

|
|
|
Periodic trends |
1. Atomic size Q .....q
Down and to the left. Periods: +proton outweighs e-s Groups: energy level increase (shield also)
2. Ionization Energy (IE)
Q ..... QQ q
|
|
|
Atomic size |
The size of an atom is half the distance between 2 nuclei in a molecule consisting of 2 identical atoms. Q ---- Q ---- = 2r r= size of one atom. |
|
|
Ionization Energy (IE) |
A measure of the amount of energy required to remove and e- from a gaseous atom or ion.
X (g) --> X^+ (g) + e- |

