![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
40 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Define: ligand, receptor.
|
ligand: ANY molec that binds a receptor/enzyme/transporter target protein
receptor: a receptor is a protein molecule, embedded in either the plasma membrane or cytoplasm of a cell, to which a mobile signaling (or "signal") molecule may attach |
|
|
|
Define: potency
|
Define: potency Potency = (EC50) the amount of drug needed to produce a particular response
|
|
|
|
Can ligands be agonists OR antagonists?
|
Yes.
Ligands... Can inactivate receptor = “antagonist” Can activate receptor = “agonist” |
|
|
|
Can receptors identify “natural” versus “synthetic” chemicals?
|
No, not if they're identical in chemical nature
|
|
|
|
List and describe the three main types of enzyme inhibitors; include whether ligand/enzyme binding is reversible, whether it is competitive, and which site binding occurs at.
|
COMPETITIVE REVERSIBLE Inh: drug binds reversibly to ACTIVE SITE
--drug inh enz activity -> stops rxn --incr subst conc can out compete inh --ex. Benazepril, captopril (ACE inhs) COMPETITIVE IRREVERSIBLE Inh: Drug binds irreversibly into ACTIVE SITE The drug inhibits the enzyme activity permanently -> stopping that reaction --Increasing substrate concentration will not out comptete inhibitor --The drug mimics the shape/properties of the substrate --ex. acetylcholinesterases NONCOMPETITIVE INH/POTENTIATOR: Drug binds into an ALLOSTERIC site --drug inhibits the enzyme activity => stopping that reaction --Potentiators increase the enzyme activity => increasing the products --Increasing substrate concentration will not compete out inhibitor --Often drug does NOT mimic the shape of the substrate |
any competitive = active site
any non-comp = allosteric site just think of two types of competitive (revers/irrevers) and non-competitive allosteric |
|
|
Most drug action at enzymes works via..., not...
|
Most drugs act via INHIBITON AT RECEPTORS, not on enzymes
|
|
|
|
Explain the characteristics of the molecular interaction between a drug and receptor during binding: including concentration dependence, binding selectivity, reversibility of binding, and how a drug stabilizes a receptor into either open/on or closed/off shapes.
|
The drug mimics the shape/properties of the body’s ligand
Drug binds into binding site on receptor (either active site or allosteric) This changes the shape of the receptor. This either ACTIVATES or BLOCKS activation of the receptor |
|
|
|
Describe the two main types of transcellular receptors and their primary (1st) actions.
|
LIGAND GATED ION CHANNELS: opens channel in receptor to let ions through (in or out)
G-PROTEIN COUPLED RECEPTORS: change in shape of receptor releases and activates G enzyme's NT (normally the Gprots are attached to the receptor and not active) |
|
|
|
Identity the three main secondary (2nd) results that the primary actions can initiate.
|
The first step can potentially activate further steps:
Activate/inhibit 1. A/I intracellular enz pathways 2. A/I ion channel opening 3. A/I gene transcription (estradiol, progestrin) |
|
|
|
Explain the three main ways to terminate a receptor signal
|
1. Unbinding of drug (followed by drug reuptake or breakdown)
2. Internalization and degradation of drug-bound receptor complex 3. Desensitization of drug-bound receptor |
|
|
|
Which two termination methods can be part of tolerance?
|
Internalization & degradation, Desensitization
|
|
|
|
Explain Desenstation
|
Desensitization is when the drug or endogenous ligand stays bound to the receptor, but the receptor switches for a while into a phase where it can no longer function. So even though the ligand is still bound, you get no effect.
|
|
|
|
Recognize the differences between the four types of secreted molecule in terms of what type of cells/tissue are affected. Be able to explain these concepts in a short answer format:
|
1. AUTOCRINE- Released onto self cell; releasing cell also expresses receptor.
e.g. Often used for inflammatory responses and in organ development, and cancer. 2. SYNAPTIC- Neurotransmitter signaling in neurons. Only cells synaptically adjoined and expressing receptor will respond. e.g. Glutamate and acetylcholine are examples 3. PARACRINE- Local cells exposed. Only cells expressing receptor will respond. e.g. Histamine 4. ENDOCRINE- Secreted into blood; cells throughout whole body are exposed. Only cells expressing receptor will respond. e.g. Estrogen. Often include intracellular “receptors” or gene transcription modulators. |
|
|
|
Affinity
|
BINDING-how well does drug attach
|
|
|
|
Potency
|
EFFECT/efficacy-how well does drug activate target
|
|
|
|
Efficacy
|
Potency
|
|
|
|
Describe the difference between affinity and potency.
|
Affinity is how easily the drug binds to target, while potency is how reliably the attachement of the drug to the receptor will result in the desired effect
|
|
|
|
Which numbers do you use to measure efficacy, potency, and affinity (Ex. KD, EC50, Emax, and Bmax)? What are the units used for each?
|
Bmax - binding (100% bound)
KD - affinity: Concentration of drug needed to produce binding to 50% of receptors (conc.) EC50 - measure of potency; the concentration of agonist producing 50% of max effect (conc.) Emax - max effectiveness of drug; how well a drug activates the receptor (whatever was measured: %, # of asthma attacks, pupil dilation mm, etc) |
|
|
|
What is the relationship between high/low affinity or potency and high/low Kd or EC50? (example: high affinity = ?)
|
low Kd/EC50 indicates a high affinity/potency
|
|
|
|
any drug that produces a large maximal effect has ...
|
high efficacy
|
|
|
|
Antagonists have an efficacy of _____.
|
zero
|
|
|
|
On which curve do we find dose binding?
|
dose/affinity curve
|
|
|
|
On which curve do we find potency and efficacy?
|
dose/effect curve
|
|
|
|
Visualize a semi-log dose/binding curve. Visualize a semi-log dose/effect curve with both a full agonist and a partial agonist. On these curves label the following: Both axes, KD, EC50, Emax, Bmax. Be able to draw these points on the appropriate curves.
|
dose-binding: Kd, Bmax, x:drug conc, y:binding
dose-effect: EC50, Emax, x:drug conc, y:effect we're measuring |
|
|
|
Can you determine affinity, potency, efficacy from a dose/binding curve? From a dose/effect curve?
|
dose binding: affinity
dose effect: potency, efficacy |
|
|
|
Which graph will always reach 100%: Dose/binding or Dose/effect?
|
Dose/binding although it's really asymptotic. Emax can be a low number and may not even be expressed as a percentage.
|
|
|
|
Define KD, EC50, Emax, and Bmax and know how to read them off a graph. Be able to compare the affinity, potency, and efficacy of two drugs based on seeing their graphs, and to identify agonists as full or partial.
|
drill
|
|
|
|
Recognize that each drug/receptor combination has its own affinity, potency and efficacy and that those will be different from another drug at the same receptor.
|
na
|
|
|
|
Characterize the types of receptor ligands: agonist, full agonist, partial agonist, allosteric potentiator, antagonist, competitive antagonist, non-competitive antagonist, and allosteric antagonist.
|
agonist - a chemical (drug or endogenous) that binds to the primary binding site and activates the receptor
full agonist - an agonist that optimally activates the receptor/gives best response possible (all bound receptors are activated) partial agonist - an agonist that suboptimally activates the receptor (not all bound receptors are activated) allosteric potentiator - antagonist - competitive antagonist - non-competitive antagonist - allosteric antagonist - |
|
|
|
When all ligands of a partial agonist are bound, why is there not a full agaonist-like effect?
|
even when bound to 100% of receptors, they do not activate all of them!
|
|
|
|
Explain which of the agonist/potentiator types: 1) binds primary versus secondary (allosteric) sites, 2) can or cannot activate receptor by itself.
|
ALLOSTERIC POTENTIATOR binds a SECONDARY SITE and does not activate on its own, but increases agonist binding or efficacy. In other words… it makes the agonist work better! Has ZERO efficacy.
|
|
|
|
Explain which of the antagonist types: 1) binds primary versus secondary (allosteric) sites, 2) are reversible, 3) can or cannot activate receptor by itself.
|
Agonist
1) binds primary ligand binding site, 2) can activate receptor Partial Agonist 1) binds primary ligand site, 2) partially activate receptor, 3) can block full agonist activity Allosteric potentiator 1) binds a secondary site 2) increases activity of main agonist 3) can not activate receptor alone Antagonist Compet - Revers - compet w/agonist - surmountable - 1o site irrevers - Irrevers - comp w/agonist - Non surmountable - 1o site Allosteric/Noncompet - revers - NO comp w/agonist - Non-surm - 2o site surmountable = outcompete/swamp it |
|
|
|
Discuss why a partial agonist can act as an antagonist in the presence of a full agonist.
|
competes with binding at 1o site
|
|
|
|
Define antagonist
|
Antagonist = a drug that blocks and prevents activation of the receptor
(has no efficacy) |
|
|
|
Being highly selective can ligands can bind >1 receptor? Can receptors can bind >1 ligand?
|
Yes in both cases. No drug is completely selective
|
|
|
|
Define synergism and additivity.
|
Define synergism and additivity. Synergism - The combined effect of two drugs is higher than the sum of their individual effects.
Additivity -The combined effect of two drugs is equal to the sum of their individual effects. |
|
|
|
Define synergism and additivity. Synergism - The combined effect of two drugs is higher than the sum of their individual effects.
Additivity -The combined effect of two drugs is equal to the sum of their individual effects. Describe drug/receptor theory and the ideas that it rests on. In what ways is it similar to/ different from herbalism or homeopathy? drug/receptor theory: 1. One-on-one interaction between molecules 2. increased dose = increased effects. |
Describe drug/receptor theory and the ideas that it rests on. In what ways is it similar to/ different from herbalism or homeopathy? drug/receptor theory:
1. One-on-one interaction between molecules 2. increased dose = increased effects. |
|
|
|
What's the difference between ligands and substrates?
|
substrates are chemically changed by the enzyme, while ligands aren’t
|
|
|
|
Describe drug/receptor theory and the ideas that it rests on. In what ways is it similar to/ different from herbalism or homeopathy?
|
need this answer
|
|
|
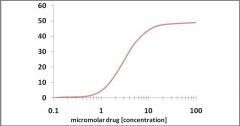
For the above dose/effect graph, which is the approximate EC50?
|
3 uM
remember that EC50 is the CONCENTRATION of drug that yields a response that's 50% of Emax |
|

