![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
76 Cards in this Set
- Front
- Back
|
orbital |
probability distribution map showing where an electron is likely to be found |
|
|
n |
principal quantum number overall size/energy n= 1,2,3,... |
|
|
l |
angular momentum number shape l= n-1 |
|
|
ml |
magnetic quantum number orientation of orbital -l to l |
|
|
ms |
spin quantum number orientation of spin + or - 1/2 |
|
|
values of l |
0- s 1- p 2- d 3- f |
|
|
each combination of n, l, and ml is |
one atomic orbital (two electrons) |
|
|
same n |
in same principal shell |
|
|
same n and l |
in same sub-shell |
|
|
energy of an orbital |
-2.18 x 10^-18 J |
|
|
change in energy |
-2.18 x 10^-18 J (1/n^2 f - 1/n^2 i) |
|
|
orbitals on periodic table |

|
|
|
periodic properties |
based on elements location |
|
|
modern periodic table |
mendeleev |
|
|
periodic law |
when the elements are arranged in order of increasing mass, certain sets of properties recur periodically |
|
|
main group elements |
properties largely predictable |
|
|
transition elements |
properties less predictable |
|
|
family/group |
column |
|
|
period |
row |
|
|
electron configuration |
particular orbitals that electrons occupy for that atom |
|
|
ground state |
lowest energy state 1s1 |
|
|
orbital diagram |

|
|
|
pauli exclusion act |
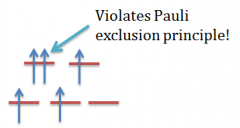
no two electrons in an atom can have the same four quantum numbers same orbital, opposite spins |
|
|
degenerate |
same energy ex: 3s,3p,3d |
|
|
coulomb's law |

potential energy of two charged particles depends on their charges and on their separation |
|
|
shielding |
innermost orbital shields outer electrons from force of nucleus |
|
|
effective nuclear charge |
net charge experienced by electrons atomic number - number of shielding electrons |
|
|
bc of penetration |
1) sublevels aren't degenerate 2) 4s is lower in energy than 3d |
|
|
aufbau principle |
electrons fill the lowest available energy levels before filling higher levels
ex: 1s before 2s |
|
|
hund's rule |
when filing degenerate orbitals, electrons fill them singly first |
|
|
valence electrons |
last s sub-shell and any f and d sub-shells that aren't filled |
|
|
s sub-shell |
2 electrons |
|
|
p sub-shell |
6 electrons |
|
|
d sub-shell |
10 electrons |
|
|
f sub-shell |
14 electrons |
|
|
group number |
number of valence electrons |
|
|
row number |
highest principle quantum number |
|
|
metals |
lower left, lose electrons |
|
|
non-metals |
upper right, gain electrons |
|
|
group 1A |
alkali metals, 1+ |
|
|
group 2A |
alkali earth metals, 2+ |
|
|
group 7A |
halogens, 1- |
|
|
group 8A |
noble gases, stable |
|
|
paramagnetic |
contains unpaired electrons |
|
|
diamagnetic |
contains only paired electrons |
|
|
covalent bonding |
nonmetals (molecular) |
|
|
metallic bonding |
metals |
|
|
ionic bonding |
metal and nonmetal (cation and an anion) |
|
|
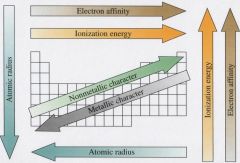
size of atoms |
cations < neutral atoms < anions |
|
|
ionization energy (IE) |
energy required to remove an electron from the atom or ion in the gaseous state |
|
|
size exceptions |
boron is smaller than beryllium aluminum is smaller than gallium |
|
|
electron affinity (EA) |
normally negative bc releases energy when gaining an electron |
|
|
metallic character increases |
moving down the periodic table |
|
|
empirical formula |
relative number of atoms (HO) |
|
|
molecular formula |
actual number of atoms (H2O2) |
|
|
duet rule |
stable lewis structure with two dots ex: He, H |
|
|
octet rule |
most stable electron configurations contain eight electrons |
|
|
element (III) |
chromium, iron, cobalt, copper, tin, mercury, lead -ous < -ic |
|
|
hydrates |
contain specific number of water molecules |
|
|
hemi |
1/2 |
|
|
mono |
1 |
|
|
di |
2 |
|
|
tri |
3 |
|
|
tetra |
4 |
|
|
penta |
5 |
|
|
hexa |
6 |
|
|
hepta |
7 |
|
|
octa |
8 |
|
|
nona |
9 |
|
|
deca |
10 |
|
|
single bond |
1 electron pair 2 electrons |
|
|
double bond |
2 electron pairs 4 electrons |
|
|
triple bond |
3 electron pairs 6 electrons |
|
|
formula mass |
average mass of a molecule =(# of atoms in 1st element x atomic mass of 1st element) + (# of atoms in 2nd element x atomic mass of 2nd element) |
|
|
composition of compounds |
mass % of element = mass of element in 1 mol/ mass of 1 mol of compound x100% |
|
|
periodic table trends
|
|

