![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
|
Ideal Gas Equation |
pV=nRT P= Pa (Pascals) V= m3 N= no. of moles R= gas constant ( J K-1 mol-1 ) T= K (temperature) |
|
|
Atom Economy |
Mr of desired product ÷ Mr of all reactants x 100 |
|
|
Rate of reaction |
Change in conc. (Moldm-3)/ time taken (s) |
|
|
Volume of gas at RTP |
Volume of gas = no. of moles x 24 |
|
|
Relative atomic mass |
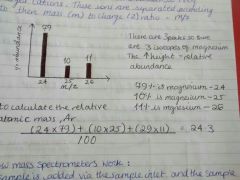
(Isotope number x abundance) + (Isotope number x abundance)/ 100 |
|
|
Speed of light |
C = wavelength (m) x frequency (s-1/ Hz) C = 3 x 10^8 ms-1 |
|
|
Energy of a photon |
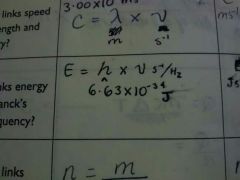
|
|
|
Percentage Yield |
Actual yield / theoretical yield x 100 |
|
|
Enthalpy change |
Enthalpy products - enthalpy reactants |
|
|
Covert gdm-3 to moldm-3 |
gdm-3 = moldm-3 X Mr |

