![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
31 Cards in this Set
- Front
- Back
|
differences between enzyme catalyzed and uncatalyzed rxns |
1. catalysis reaches saturation 2. enzymes reduce the activation energy |
|
|
Reaction Order |
refers to the number of molecules that need to interact |
|
|
Vmax |
theoretical maximum velocity of the rxn |
|
|
Km |
[S] at which half the maximal velocity is achieved. Includes the affinity of the enzyme for the substrate and the rate of conversion of ES to E+P |
|
|
Differences between steady state and equilibrium |
1. there is only one equilibrium point (when ∆G=0) |
|
|
steady state assumption |
[ES] is constant over time |
|
|
Michaelis-Menton Equation |
v₀=Vmax*[S]/{Km+[S]} |
|
|
kcat |
turnover number or catalytic constant. rate of ES→E+P. the number of reactions that an enzyme preforms per second. |
|
|
catalytic efficiency |
kcat/Km |
|
|
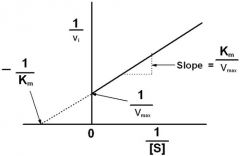
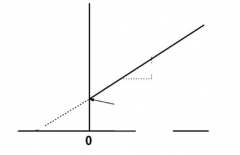
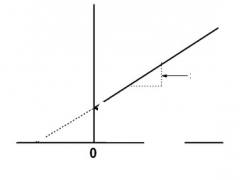
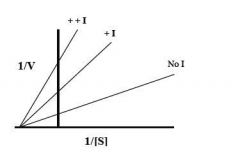
Lineweaver-burke plot |
|
|
-1/Km |
|
|
1/[S] |
|
|
1/v |
|
|
1/Vmax |
|
|
Km/Vmax |
|
|
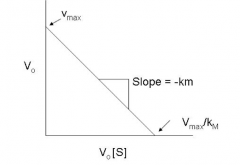
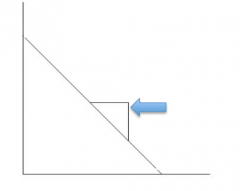
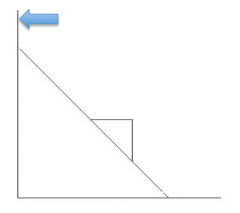
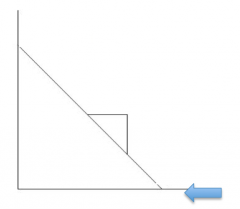
Eadie-Hofstee Plot |
|
|
Competitive Inhibitor |
|
|
Noncompetitive Inhibitor |
|
|
-Km |
|
|
v0 |
|
|
v0/[S] |
|
|
Vmax |
|
|
Vmax/Km |
|
|
Factors that effect enzymes |
pH ionic strength temperature inhibitors |
|
|
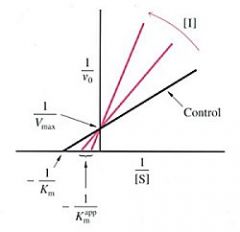
Competitive inhibtion |
inhibitor binds reversibly to the same site as the substrate Vmax is unchanged Km changes with [I]
|
|
|
Mixed (noncompetitive) inhibition |
inhibitor binds both E and ES Vmax changes with [I] Km remains constant does not affect of substrate binding |
|
|
sequential bisubstrate reaction |
both substrates first bind E then products are released. can be ordered or random. |
|
|
ping-pong bisubstrate reaction |
the product of the first substrate is released before the second substrate binds. necessitates a modified enzyme intermediate. |
|
|
oligomeric allosteric enzymes |
binding ligand by one subunit makes it easier for others to bind (cooperativity). these don't follow michaelis-menten; they have sigmoidal graphs. |
|
|
the hill equation |
log[v/(Vmax-v)]=n*log[S]-logK' n=index of cooperativity=hill coefficient |
|
|
Kd |
Measure of affinity of the ligand for the protein 1/Ka=[L][P]/[LP] |

