![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
54 Cards in this Set
- Front
- Back
|
Principles of the pituitary function tests
|
1. Measure basal pituitary hormone and target organ hormone levels.
2. Stimulation test if inadequacy suspected 3. Suppression test if excess suspected |
|
|
What is the schematic for the H-P-Target Organ system?
|
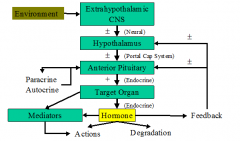
|
|
|
What is the H-P-Thyroid system? What are the specifics?
|
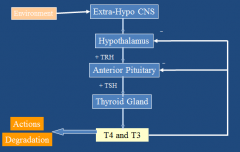
TRH: thyrotropin releasing hormone
TSH: thyroid stimulating hormone |
|
|
What is primary thyroid failure?
|
Low free T4, high TSH
Ex: lymphocytic thyroiditis |
|
|
What is 2ary thyroid failure?
|
Low free T4, low or normal TSH
Ex: Pituitary tumor |
|
|
What is the H-P-Ovarian Axis?
|
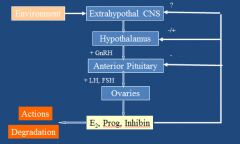
Primary ovarian failure: low estradiol, high LH/FSH
Ex: menopause 2ary ovarian failure: low estradiol, low/normal LH and FSH Ex: Pituitary tumor |
|
|
What is the H-P-Testes axis?
|
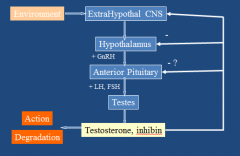
|
|
|
How do pulsatile and constant GnRH affect LH and FSH levels?
|
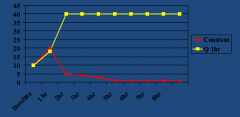
-Pulsatile: stimulates LH and FSH, used in pts w/ hypothalamic lack of GnRH (Kallman's Syndrome). LH and FSH in turn stimulate testes and increases testosterone and sperm production.
-Constant: suppresses LH and FSH after initial stimulation, which suppresses testes and decreases testosterone and sperm production. Occurs in idiopathic central precocious puberty and hormone-sensitive tumors (prostate/breast). |
|
|
Describe the H-P-Adrenal Axis.
|
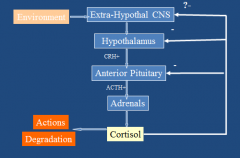
|
|
|
Describe normal cortisol secretion.
|
Highest right before we wake, lowest right before we go to bed.
|
|
|
How do the various ACTH and cortisol diseases relate to each other?
|
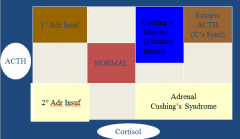
|
|
|
What does the Acute ACTH stimulation test cause? What does it show?
|
-Primary adrenal insufficiency (addison's disease): no increase in cortisol
-2ary adrenal insufficiency: +/- cortisol increase |
|
|
What does the dexamethasone suppression test show?
|
Dexamethasone is an active synthetic steroid (ACTH) not picked up by cortisol assay. In normal people, Dexa causes decreased cortisol. In Cushing's, cortisol is not decreased.
|
|
|
What is Cushing's Disease?
|
Pituitary-tumor induced Cushing's Syndrome. Tumors may be too small/amorphous to be seen clearly on pituitary imaging. Lung carcinoid tumors may secrete ACTH and mimic Cushing's Disease.
|
|
|
Describe the control of aldosterone
|
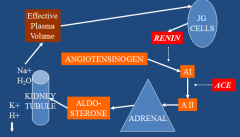
|
|
|
Which hormones affect the gluco- and mineralo-corticoids?
|
Gluco: ACTH. Replace glucocorticoids if pit def.
Mineralo: renin/angiotensin. Replace gluco and mineralo if adrenal def. |
|
|
Describe the H-P-Growth hormone axis.
|
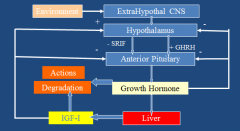
-GH secretion is diurnal, w/ highest levels soon after sleep starts. Get a second peak soon after waking. Is pulsatile and changes w/ aging.
-Direct Effects: most metabolic effects, feedback -Indirect Effects: Most growth effects, mediated by IGF-1, feedback -Acute Effects: increase fat synthesis, increase glucose utilization -Chronic effects: decrease fat synthesis and glucose utilization, increase protein synthesis and gluconeogenesis. -GH secretion stimulation: Insulin-induced hypoglycemia, exercise, arginine, GHRH, glucagon. |
|
|
Why/how is IGF-1 used as a screening test for growth hormone?
|
-high in acromegaly, low in hypopituitarism.
-IGF-1 affected by age, sex, nutrition -Not affected by time of day, stress, etc. -not adequate as definitive test. |
|
|
Describe the H-P-Prolactin axis.
|
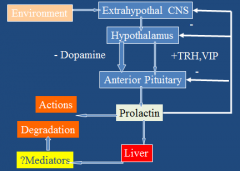
-Prolactin feeds back on own seretion via dopamine. If connection btwn hypothalamus and ant pit severed, all hormones decrease except prolactin.
-Get increase in hormone levels 30secs after suckling starts. -Direct Effects: lactation, immune effects (?), effects on fat and adipokines. -Indirect effects: mediation by IGF-1 |
|
|
What is Whipple's Triad?
|
To be hypoglycemic, must have all 3:
1. Plasma glucose < 45mg/dL 2. Concomitant sx 3. Relief of sx following carbs. |
|
|
What is the hierarchy of response to hypoglycemia?
|
Plasma glucose (mg/dL)
70: catecholamines, glucagon 60: cortisol, GH 50: Autonomic sx 40: Neuroglycopenic sx 30: Lethargy 20: seizures, coma |
|
|
Differentiate between autonomic and neuroglycopenic sx.
|
Autonomic: tremors, nervousness, hunger, anxiety, palpitations, diaphoresis
Neuroglycopenic: visual disturbances, lethargy, confusion, impaired performance |
|
|
Which glucose transporters are used in the CNS?
|
GLUT1: to cross BBB
GLUT3: to enter neuron |
|
|
How does the CNS react to hypoglycemia?
|
-Acute: increases cerebral blood flow to get more glucose and alleviate sx. Increase in epinephrine release
-Chronic: 50% increase in GLUT-1, possible increase in GLUT-3. decrease in epinephrine release bc brain gets used to low glucose state. |
|
|
If you do not have glycogen in your liver, can you respond to glucagon?
|
No, glucagon triggers glycogenolysis in the liver.
|
|
|
Describe the 2 types of reactive hypoglycemia.
|
-Alimentary: people think they are hypoglycemic 2-3 hrs after a meal. Occurs after gastric surgery, may be related to islet proliferation (beta and ductal cells).
-Nesidioblastosis: hypoglycemia that occurs after bypass surgery. |
|
|
How do you tx reactive hypoglycemia?
|
1. alpha-glucosidase inhibitors work in most people
2. Diazoxide to decrease insulin secretion 3. avoid caffeine 4. rare - pancreatectomy |
|
|
What are the 7 causes of spontaneous hypoglycemia?
|
ONI HEAD: Organ failure, non-beta cell tumors, insulin secreting tumors, hormone deficiencies, enzyme deficiencies, autoimmune, drugs
|
|
|
Describe insulin-induced hypoglycemia.
|
-relatively common
-more frequent in DM1 who lack glucagon response to hypoglycemia. -more common in pts w/ hypoglycemia unawareness. |
|
|
How does EtOH cause hypoglycemia?
|
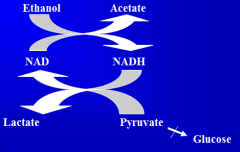
-EtOH inhibits gluconeogenesis. Alcoholics usually don't eat, are in starvation, and glycogen is depleted, so can't respond to glucagon. EtOH also impairs the cortisol and GH response to hypoglycemia.
-EtOH also actively inhibits gluconeogenesis. -To break down EtOH, pyruvate is not made, can't do gluconeogenesis, lactate builds up. Further can't release glucose from liver. |
|
|
How do salicylates cause hypoglycemia?
|
-Inhibit FFA oxidation, impairing gluconeogenesis.
-Also inhibits serine kinase IKK-beta -> increased insulin sensitivity -> hypoglycemia |
|
|
How does caffeine affect cerebral blood flow?
|
Caffeine decreases cerebal blood flow, so brain cant compensate as much to hypoglycemia. brain sees more hypoglycemia, get greater symptoms and release of epi.
|
|
|
How do beta blockers cause hypoglycemia?
|
Lowers the threshold for autonomic sx in poorly controlled pts w/ DM1, so sx used to start at 80 mg/dl, now start at 60. Have minimal decrease in adrenergic sx and increased cholinergic sx. Harder for them to recognize their hypoglycemic sx.
|
|
|
What are the different enzyme deficiencies that can cause hypoglycemia?
|
-defects in fatty acid oxidation
-G6P deficiency, glycogen storage disease -Galactosemia -Inborn errors of amino acid metabolism |
|
|
How do non-beta cell tumors cause hypoglycemia?
|
they are very large, and assoc w/ increased secretion of IGF-2, which circulates at lower molecular weight than IGF-1, permitting greater tissue accessibility.
|
|
|
How do Insulinomas cause hypoglycemia?
|
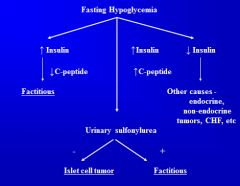
-very rare
-Do multiple tests -Insulin remains high even when fasting, have decreased glucose utilization. -Best way to find is via arteriography w/ Ca infusion |
|
|
How do hypoglycemia and HbAlc levels relate?
|
Higher HbA1c = more risk of hypoglycemic episodes, but have more severe hypoglycemic episodes with lower HbA1c.
|
|
|
Describe hypoglycemia in diabetics.
|
-Type 1 has more episodes with increased severity.
-Type 2 has fewer bc of greater preservation of counterregulatory response to early hypoglycemia, such as glucagon. -Tx: 15-25 gm carbs. -Sulfonylurea can prolong hypoglycemia, need to be hospitalized for 24 hrs, until drug is out of system. |
|
|
Describe how glucose causes insulin secretion.
|
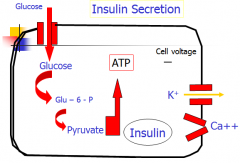
In basal state, ATP dep K channel is open, K runs out, makes cell negative. Ca channels are closed. When we eat, glucose is metabolized, makes ATP, ATP binds to K channel, closes it, cell becomes depolarized/positive bc K not leaving. Ca channel then opens after depolarization. Ca entering cell causes release of insulin.
|
|
|
How does GLP-1 cause insulin secretion?
|
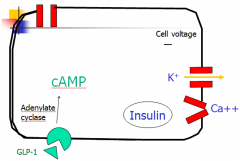
GLP-1 bings to receptor, AC turns ATP into cAMP, which binds to K channel, closing it. Depolarization of cell causes Ca channel to open and Ca to enter cell. cAMP and Ca both act to release insulin.
|
|
|
How does insulin lead to glucose uptake?
|
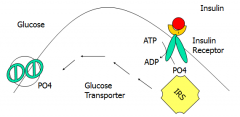
Insulin binds to extracellular alpha subunit of receptor. Transmembrane portion is a kinase, and phosphorylates self by making ATP -> ADP. This starts a kinase cascade, which includes IRS. Phosphorylation of GLUT4 leads to its translocation to the plasma membrane.
|
|
|
What happens when there is no insulin or insulin resistance?
|
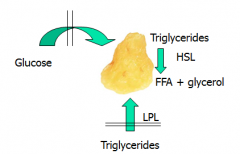
No glucose can be taken up by muscles, so think you are hypoglycemic. Get glycogen breakdown and protein degradation. Leads to increased lactate, aa's, and alanine in blood.
-Fat: LPL is insulin sensitive and in bloodstream, so not able to move triglycerides into adipose cells. HSL is sensitive to cortisol, catecholamine, glucagon, -> causes release of TAGs into blood. LIPOLYSIS. -Overall: glycogenolysis, gluconeogenesis, ketogenesis (from FFAs), increased hepatic glucose output. |
|
|
How is glucagon secreted by the alpha cells?
|
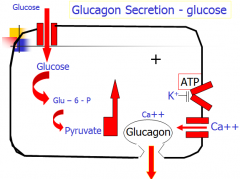
Glucose enters cell, made into pyruvate to make ATP. ATP closes K channel, cell depolarizes, Ca enters, glucagon leaves.
|
|
|
How does insulin influence glucagon secretion?
|
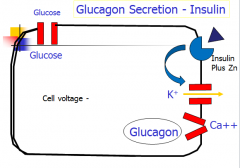
If present, insulin + Zn will bind to separate receptor and keep K channel open, so cell cannot depolarize.
|
|
|
How does epinephrine affect glucagon secretion?
|
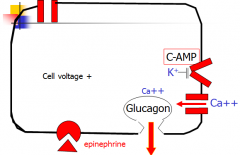
Epi binds to receptor, makes cAMP, cAMP binds to K channel, cell depolarizes, Ca enters, glucagon leaves.
|
|
|
What scenarios cause glucagon release?
|
1. Hypoglycemia - islet interstitial conc of insulin low, or release of catecholamines.
2. Diabetes induced hyperglycemia - insulin conc too low or alpha cell is insulin resistant |
|
|
How does glucagon increase ketogenesis?
|
1. Stimulates hormone sensitive lipase (HSL) to make more FFAs
2. Inhibits acetyl coA carboxylase to decrease malonyl coA resulting in more fatty acid oxidation 3. Stimulates ketogenic enzymes (HMG-CoA synthase and lyase). |
|
|
What are the consequences of hyperglycemia?
|
1. osmotic diuresis: pee out glucose, fluids, electrolytes
2. Urinary loss of water, electrolytes leave w/ glucose, get dehydrated 3. Weakness, weight loss, shock b/c can't maintain BP. |
|
|
What are the consequences of ketosis?
|
-Acidosis
-impaired cardiovascular fxn. |
|
|
What are the differences between DKA and non-ketotic comas?
|
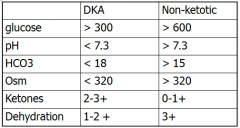
in DKA - usually sick for some other reason, not as severe as ketotic, but comes on much quicker, w/in hrs. Osm higher in non-ketotic bc of higher glucose. Also have much more renal insufficiency in non-ketotic coma.
|
|
|
What are the diagnostic criteria for diabetes?
|
1. Presence of classic sx plus a casual plasma glucose > 200mg/dL
2. Fasting plasma glucose > 126mg/dL 3. 2 hr plasma glucose >200 mg/dL during OGTT 4. HbA1c > 6.5% |
|
|
What is the diagnostic criteria for gestational diabetes? Why is is stricter?
|
Stricter bc of risk of fetal loss and/or malformations.
1. Initial screen >140mg/dl one hr after 50g glucose load 2. Diagnosis: fasting greater than 95, 1 hr greater than 180, 2 hr greater than 155, 3 hr greater than 140 during a 100g glucose challenge. |
|
|
What is the criteria for impaired glucose tolerance?
|
1. Fasting plasma glucose btwn 100 and 126.
2. 2hr post glucose load btwn 140 and 200. 3. Not assoc w/ microvascular complications of DM but at risk for developing DM. 4. Assoc risks of insulin resistance syndrome. |
|
|
What is the best clinical parameter for differentiating btwn type 1 and type 2?
|
Type 1 is ketoacidosis prone.
|

