![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
318 Cards in this Set
- Front
- Back
|
What kind of test can you consider pregnancy?
|
Stress test of maternal physiology - every organ needs to work harder to meet the demands of the growing fetus and to prepare/protect from CV injury at time of delivery
|
|
|
What happens when an organ system is unable to meet the increased physiologic demand of pregnancy?
|
Gestational syndromes develop in previously healthy women (typically transient and resolve after delivery)
|
|
|
What do some gestational syndromes (inability of organ system to meet increased physiologic demands of pregnancy) tell you?
|
These may either predispose or predict the reappearance of related physiologic disorders in later life (eg, gestational diabetes is often predictive of diabetes mellitus later in life)
|
|
|
How are blood glucose levels affected by pregnancy compared to normal?
|
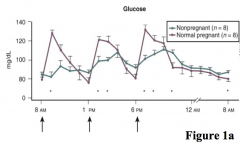
Relative to non-pregnant woman:
- Elevated in fed state - Decreased during fasting |
|
|
How are insulin levels affected by pregnancy compared to normal?
|
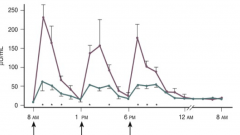
- Hyperinsulinemia: elevated insulin levels relative to non-pregnant women
- Development of insulin resistance |
|
|
How do you assess insulin resistance in a patient? How is resistance affected by pregnancy?
|
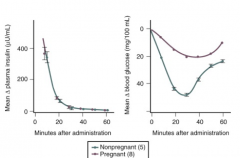
- Insulin Tolerance Test: give standard dose of insulin followed by serial blood glucose measurements
- Pregnant: smaller decrease in circulating glucose after insulin given |
|
|
How are blood lipid levels affected by pregnancy compared to normal?
|
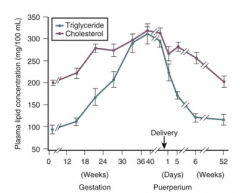
- Hyperlipidemia (TGs, cholesterol, FFAs) in fed and fasting state
- Hypertriglyceridemia in fed state - Plasma lipid concentration progressively increases after 24 weeks gestation |
|
|
How are blood ketone body levels affected by pregnancy compared to normal?
|
Hyperketonemia = increased circulating levels of ketone bodies
|
|
|
Why is it beneficial to have increased production of lipolytic hormones during pregnancy?
|
These hormones mobilize FFAs to serve as an energy source for the mother
|
|
|
What happens to the maternal primary source of energy throughout pregnancy?
|
- Switches from carbohydrates to fats as primary energy source
- Mother uses TGs, glycerol, and FFAs as metabolic fuels after meals, leaving glucose and amino acids for fetus - Protein catabolism is minimized to preserve muscle mass |
|
|
What relative change happens to the following levels during pregnancy:
- Glucose - Insulin - Glucagon - Amino Acids - Alanine - FFAs - Cholesterol |
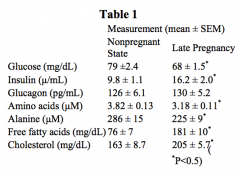
- Glucose: ↓
- Insulin: ↑ - Glucagon: ≈ - Amino Acids: ↓ - Alanine: ↓ - FFAs: ↑ - Cholesterol: ↑ |
|
|
What is the definition of Gestational Diabetes (GDM)?
|
Onset or first recognition of abnormal glucose tolerance during pregnancy (but not previously undiagnosed diabetes that is diagnosed at first pre-natal visit)
|
|
|
How common is Gestational Diabetes (GDM)? Which ethnic groups have a higher prevalence?
|
- ~7% of all pregnancies
- Higher in African Americans, Hispanic Americans, Native Americans, Pacific Islander, and Asian American women |
|
|
How does a pregnant woman try to compensate for increased peripheral insulin resistance?
|
- Peripheral insulin resistance increased during pregnancy
- Pancreatic β-cells respond by increasing their mass and with hyper-secretion of insulin |
|
|
What happens to the compensatory mechanisms of a pregnant woman that leads to Gestational Diabetes (GDM)?
|
Insufficient compensatory capacity to increase β-cell mass and insulin secretion
|
|
|
What can contribute to the pathophysiology of developing Gestational Diabetes (GDM)?
|
- Pre-existing insulin resistance d/t obesity
- Existence of circulating islet-cell antibodies ("latent" form of T1DM), in ~10% of women with GDM |
|
|
What are the criteria for being considered a low-risk patient for Gestational Diabetes (GDM)?
|
All of the following apply to be considered low-risk:
- Age < 25 years - Weight normal before pregnancy - Weight normal at birth (between 6-9 pounds) - No history of abnormal glucose metabolism - No history of poor pregnancy outcome or over weight baby (>9 pounds) - No known diabetes in first-degree relatives - Member of an ethnic group w/ a low prevalence of GDM |
|
|
What are the criteria for being considered a high-risk patient for Gestational Diabetes (GDM)?
|
One or more of the following:
- Severe obesity (BMI > 40) - Family history of diabetes, especially in first degree relatives - Previous history of GDM - Member of an ethnic group w/ higher than background rates of T2DM |
|
|
If you designate a patient as "high risk" for Gestational Diabetes (GDM), what do you need to do?
|
Screen by blood glucose testing as soon as feasible
|
|
|
If you designate a patient as "low risk" for Gestational Diabetes (GDM), what do you need to do?
|
You don't need to do anything until 24-28 weeks gestation, when you should screen for Gestational Diabetes (GDM)
|
|
|
How do you diagnose Gestational Diabetes (GDM)?
|
At least two abnormal tests:
- Fasting glucose and/or - Glucose tolerance tests after 1 or 2 or 3 hours of glucose ingestion Fasting plasma glucose: 95-126 mg/dL (if >126 mg/dL than it is overt diabetes) Glucose Tolerance Test: - 1 hr: >180 mg/dL - 2 hr: >155 mg/dL - 3 hr: >140 mg/dL |
|
|
What results for a fasting plasma glucose test are suggestive of Gestational Diabetes (GDM)?
|
Between 95-126 mg/dL
(If >126 mg/dL than it is overt diabetes) |
|
|
What results for a glucose tolerance test are suggestive of Gestational Diabetes (GDM)?
|
After 75 g oral glucose:
- 1 hr: >180 mg/dL - 2 hr: >155 mg/dL - 3 hr: >140 mg/dL |
|
|
What are the risks if your patient is diagnosed with overt diabetes at your first prenatal visit (fasting glucose >126 mg/dL)?
|
Increased risk for spontaneous abortion and congenital anomalies:
- Pre-eclampsia - Macrosomia (elevated birth weight) - Birth trauma - Necessity of C-section - Polyhydramnios (too much amniotic fluid) - Stillbirth - Neonatal hypoglycemia - Respiratory distress |
|
|
What are congenital anomalies related to Gestational Diabetes (GDM) linked to?
|
Congenital anomalies linked to poor glycemic control during first 7 weeks post-conception when most of fetal organogenesis occurs
|
|
|
What are the long-term complications to the mother with Gestational Diabetes (GDM)?
|
- 10% increased likelihood of overt DM immediately after pregnancy and 40% within 20 years
- Women w/ circulating islet-cell antibodies are predisposed to T1DM after delivery (risk dependent on HLA type) |
|
|
What are the long-term complications to the offspring with Gestational Diabetes (GDM)?
|
8-fold higher risk of diabetes or pre-diabetes at 18-27 years of age
|
|
|
How do you manage Gestational Diabetes (GDM)?
|
Maintain good glycemic control
- Medical nutritional therapy within 1 week of GDM diagnosis w/ a minimum of 3 nutrition visits - Must monitor their blood glucose, including fasting and post-prandial levels |
|
|
What does increased obesity in pregnancy correlate with?
|
Positive relationship between increased BMI and risk for:
- Pre-eclampsia - Gestational HTN - Caesarean delivery - Still-birth - Large-for-gestation age infants |
|
|
What is the relative risk of adverse outcomes in previously obese women who have undergone bariatric (weight loss) surgery?
|
Reduced risk of adverse outcomes
|
|
|
What is the capacity for facilitated transfusion of glucose from maternal to fetal circulation? Implications?
|
- High placental capacity, saturated at only ≥20 mmol/L
- Small changes in maternal circulating glucose levels are transmitted to fetus - Excess nutrient transfer to fetus results in fetal over-growth and increased adiposity (generates a cross-generational cycle of excessive weight gain and metabolic syndrome) |
|
|
How does obesity affect Gestational Diabetes (GDM)?
|
- Obese women have higher circulating glucose
- Obesity exaggerates insulin resistance |
|
|
What is increased in obese pregnant women relative to healthy weight women?
|
- Increased circulating glucose
- Increased insulin resistance - Hyperinsulinemia - Increased circulating inflammatory cytokines |
|
|
How common are hypertensive disorders in pregnancies? Most common cause?
|
- 7-10% of all pregnancies
- Approximately 70% d/t gestational HTN, which includes pre-eclampsia and eclampsia |
|
|
What is the leading cause of maternal morbidity and mortality?
|
Eclampsia
|
|
|
What is a variant of pre-eclampsia? Symptoms?
|
HELLP:
- Hemolysis w/ a microangiopathic blood smear - Elevated Liver enzymes - Low Platelet count |
|
|
What are both severe pre-eclampsia and HELLP syndrome associated with?
|
Serious hepatic manifestations, including infarction, hemorrhage, and rupture
|
|
|
What happens to most patients with pregnancy-associated hypertension once they give birth?
|
Most women (85%) with pregnancy-associated HTN become normotensive by 12th postpartum week
|
|
|
How prevalent is Pre-Eclampsia?
|
- 7.5% of all pregnancies
- 15-25% of women initially diagnosed with gestational HTN |
|
|
Is pre-eclampsia more likely to present early or late?
|
Late onset pre-eclampsia (>34 weeks) is more prevalent than early onset disease
|
|
|
How prevalent is HELLP?
|
- Approximately 0.1-0.8% of all pregnancies
- 10-20% of women with severe pre-eclampsia / eclampsia |
|
|
What are the diagnostic criteria for gestational HTN?
|
In a previously normotensive pregnant women, who is >20 weeks of gestation and has NO proteinuria or new signs of end-organ dysfunction:
- Systolic BP > 140 mmHg and/or - Diastolic BP > 90 mmHg Should be documented at least 2x, at least 4+ hours apart |
|
|
When is gestational HTN considered severe?
|
Sustained elevations in Systolic BP (>160 mmHg) and/or Diastolic BP (>110 mmHg) for at least 4 hours
|
|
|
What kind of diagnosis is Gestational HTN?
|
Temporary diagnosis for hypertensive pregnant women who do not meet criteria for pre-eclampsia or chronic HTN
|
|
|
When is a pregnant woman considered to have "chronic HTN" (as opposed to gestational HTN)?
|
Chronic HTN is diagnosed before the 20th week of pregnancy)
|
|
|
What happens to a patient with gestational HTN after giving birth?
|
Transient gestational HTN will return to normal by 12 weeks post-partum
|
|
|
What are the diagnostic criteria of Pre-Eclampsia?
|
- Systolic BP > 140 mmHg and/or
- Diastolic BP > 90 mmHg AND - Proteinuria and/or end-organ dysfunction (10% will have no proteinuria) Should be documented at least 2x, at least 4+ hours apart |
|
|
Do you have to have proteinuria to be diagnosed with Pre-Eclampsia?
|
No, 10% of women with clinical and/or histological manifestations of pre-eclampsia have no proteinuria but they do have end-organ dysfunction (which is managed as pre-eclampsia)
|
|
|
Besides the BP and proteinuria, what other changes are consistent w/ pre-eclampsia?
|
- Thrombocytopenia (platelet counts <100,000/µL)
- Increased creatinine concentration (>1.1 mg/dL) - Doubling of hepatic transaminases, ALT and AST (abnormal liver function) - Headaches - Epigastric pain - Visual disturbances |
|
|
What are the potential maternal sequelae/consequences of pre-eclampsia?
|
- Pulmonary edema
- Cerebral hemorrhage - Hepatic failure - Renal failure - Development of seizures and death |
|
|
What are the potential fetal consequences of pre-eclampsia?
|
- Placental hypo-perfusion
- Frequently need early delivery |
|
|
What are the diagnostic criteria for Eclampsia?
|
*Development of seizures in woman with pre-eclampsia, in the absence of other neurological conditions that could account for seizure*
- Systolic BP > 140 mmHg and/or - Diastolic BP > 90 mmHg AND - Proteinuria and/or end-organ dysfunction (10% will have no proteinuria) Should be documented at least 2x, at least 4+ hours apart |
|
|
How often do patients with HELLP syndrome (Hemolysis, Elevated Liver enzymes, and Low Platelet count) present without antecedent HTN or proteinuria (pre-eclampsia)?
|
15-20%
|
|
|
Pre-eclampsia is a disease of what organ?
|
Placenta
- It resolves after delivery, but persists in rare situations where removal of placenta is not possible at time of delivery |
|
|
What are the components of the pathogenesis of Pre-Eclampsia?
|
Poorly understood:
- Shallow placentation or incomplete spiral artery remodeling - Defective trophoblast differentiation - Placental hypo-perfusion - Aberrant expression / release of anti-angiogenic factors (sFlt1 and sEng) by the placenta - Angiotensin receptor auto-antibodies and other immunological mechanisms |
|
|
What are spiral arteries?
|
Terminal branches of the uterine artery that supply blood to the placenta
|
|
|
What is the effect of shallow placentation or incomplete spiral artery remodeling?
|
Mechanism of pathophysiology leading to Pre-Eclampsia:
- Normally, spiral arteries are transformed from muscular arterioles to low resistance large capacitance vessels - Essential for sufficient blood supply to placenta |
|
|
What is the effect of defective trophoblast differentiation?
|
Mechanism of pathophysiology leading to Pre-Eclampsia:
- Normally, endovascular trophoblast cells (those that invade BVs) go through pseudo-vasculogenesis where they switch adhesion molecules from those characteristic of epithelial cells to those of endothelial cells - This is incomplete or not observed in pre-eclampsia |
|
|
What is the effect of placental hypoperfusion?
|
Mechanism of pathophysiology leading to Pre-Eclampsia:
- Pre-eclampsia is more common at higher altitudes - Pre-eclampsia can be reproduced in animals by reducing placental blood flow |
|
|
Which anti-angiogenic factors are released by the placenta? Implications?
|
- sFlt1 and sEng
- Aberrant expression is associated with Pre-Eclampsia |
|
|
What immunological mechanisms may contribute to the development of Pre-Eclampsia?
|
Angiotensin receptor (AT1) auto-antibodies and other mechanisms relevant to some patients:
- Increased activation of AT1 by auto-antibodies could induce HTN and vascular injury |
|
|
What is the key feature in the pathophysiology of pre-eclampsia?
|
Hypoperfusion and ischemia of placenta
|
|
|
What are the risk factors for Pre-Eclampsia?
|
- Primiparity (first pregnancy)
- Past history of pre-eclampsia (7-fold increased risk) - Family history of pre-eclampsia in first degree relative of mother or father - Pre-existing medical conditions such as diabetes, high BMI (>26.1), CKD, or anti-phospholipid antibodies - Twin pregnancies or more fetuses - Maternal age < 20 or >35 - Women with early onset gestational HTN - Increase in sFlt-1 levels |
|
|
What ages are associated with increased risk of Pre-Eclampsia?
|
< 20 years
> 35 years |
|
|
Which pre-existing medical conditions are associated with a higher risk of pre-eclampsia?
|
- Diabetes
- High BMI (>26.1) - Chronic Kidney Disease - Anti-Phospholipid Antibodies |
|
|
How do you manage patients with hypertensive disorders in pregnancy?
|
- Closely monitor w/ weekly out-patient visits (in non-severe gestational HTN)
- Monitor BP 1-2x/week - Weekly assessment of proteinuria, platelet counts, and liver enzymes - Patient education and counseling to identify symptoms of severe disease and signs of possible fetal/placental impairment - Fetal well-being assessed |
|
|
How should you assess fetal well-being in patients with any gestational hypertensive disorder?
|
- Biophysical profile (fetal muscle tone and movement assessment
- Non-stress test (fetal HR and uterine contractions) w/ amniotic fluid estimation - Sonographic estimation of fetal weight - Umbilical artery doppler velocimetry for growth restricted fetuses |
|
|
What patient education and counseling should you give a patient with a hypertensive disorder?
|
- Identify symptoms suggesting severe disease (severe headache, visual changes, epigastric or RUQ pain)
- Identify signs of possible fetal/placental impairment (decreased fetal movement, vaginal bleeding, signs of pre-term labor) - Given appropriate numbers to call providers for questions and emergencies |
|
|
Can you prevent Pre-Eclampsia? If so, how?
|
- No proven preventative therapy for pre-eclampsia
- The only cure is delivery - Anti-HTN agents are not used in non-severe gestational HTN |
|
|
How do you cure Pre-Eclampsia?
|
Delivery (based on disease severity, presence of co-morbidities, and risk of adverse pregnancy outcome)
|
|
|
How do you manage a patient with pre-eclampsia during labor?
|
- Monitor for worsening HTN and symptoms of severe pre-eclampsia
- May need to manage with anti-HTN therapy - May need to use Magnesium Sulfate for seizure prophylaxis (magnesium toxicity leads to loss of deep tendon reflexes and respiratory depression) - Actively bleeding patients with thrombocytopenia may need platelet transfusion - Two injections of Betamethasone (24 hrs apart) may be given 48 hours prior to delivery to speed up fetal lung maturation if delivery needs to be done <34 weeks) |
|
|
What should you do if delivery is necessary prior to 34 weeks of gestation? Function?
|
- 2 injections of Betamethasone (24 hours apart) 48 hours prior to delivery
- Speeds up fetal lung maturation |
|
|
What are the pregnancy outcome and long-term prognosis for a mother with gestational HTN / pre-eclampsia?
|
- Increased risk of developing any of these disorders in future pregnancies (recurrence highest for women w/ more severe and w/ early-onset)
- Increased risk of developing HTN and disease related to HTN (CV disease, kidney disease, DM) later |
|
|
What are the pregnancy outcome and long-term prognosis for a fetus with gestational HTN / pre-eclampsia?
|
- Fetal growth restriction (associated w/ long-term CV risk for baby)
- Oligohydramnios (reduced amniotic fluid) - Indicated pre-term delivery and its sequelae |
|
|
What is the greatest adverse effect on BW and fetal well-being?
|
Severe early onset pre-eclampsia (risk of fetal death is increased by 5-fold and risk of perinatal death or severe morbidity by 16-fold)
|
|
|
What are the pregnancy associated changes in thyroid physiology?
|
- Elevated circulating TBG (thyroid-binding globulin) by 1.5 fold
- Decreased TSH production during first trimester but increased later - Increased T3 and T4 production |
|
|
What happens to the relative level of Thyroid Binding Globulin (TBG) during pregnancy? Why?
|
- TBG is elevated 1.5 fold starting at 6-8 weeks and remains elevated
- Increased production and decreased clearance, mediated by increased in estrogen - TBG synthesized in liver and carries T3 and T4 |
|
|
What happens to the relative level of Thyroid Stimulating Hormone (TSH) during pregnancy? Why?
|
- TSH production goes down in 1st trimester (d/t increased hCG production)
- Later in pregnancy it increases (when hCG falls) - β-subunit of hCG is homologous to β-subunit of TSH and has weak ability to stimulate TSH receptor (thus when more hCG, less TSH, and vice versa) |
|
|
What happens to the relative level of free Thyroid Hormones during pregnancy? Why?
|
- T3 and T4 production increases in 1st half, but plateaus at 20 weeks when rate of thyroid hormone production returns to pre-pregnancy state
- Via action of hCG in first trimester and through increased TSH later in pregnancy |
|
|
What is the most reliable indicator of gestational thyroid status? Less reliable?
|
* Most reliable: maternal serum TSH levels
- Free T3 and T4 measures are unreliable d/t high circulating TBG and decreased albumin - Total T3 and T4 can be measured, and upper limit of 1.5x higher than non-pregnant reference range may be used |
|
|
What are the recommended ranges for TSH during pregnancy?
|
- 1st trimester: 0.1-2.5 mIU/L
- 2nd trimester: 0.2-3.0 mIU/L - 3rd trimester: 0.3-3.0 mIU/L (remember TSH production goes down during 1st trimester b/c hCG is able to weakly activate the TSH receptor) |
|
|
What is the definition of hypothyroidism in pregnancy? How common?
|
- Elevated TSH level w/ normal free circulating T4
- 2-3% of pregnant women have undiagnosed hypothyroidism and 2/3 of those are sub-clinical |
|
|
What is the most common cause of hypothyroidism world-wide?
|
Iodine deficiency (higher requirements in pregnancy)
|
|
|
What are the associated changes in severe iodine deficiency in pregnancy?
|
- Resultant hypothyroidism
- Neurocognitive impairment and mental retardation in offspring |
|
|
When iodine intake is adequate, what is the most common cause of hypothyroidism in pregnancy?
|
Presence of Thyroperoxidase (TPO) or Thyroglobulin auto-antibodies
- TPO: thyroid enzyme that adds iodine to thyroglobulins (in colloid) in generation of T3 and T4 |
|
|
How common are auto-antibodies to Thyroperoxidase (TPO) or Thyroglobulin in women of child-bearing age? What disease?
|
10-20% of women have these auto-antibodies
- Indicates Hashimoto's Thyroiditis, which leads to auto-immune destruction of thyroid gland |
|
|
How severe is hypothyroidism d/t Hashimoto's Thyroiditis in pregnant women?
|
- In majority, degree of thyroid destruction is not sufficient to cause hypothyroidism
- A significant association is observed with pregnancy loss, recurrent miscarriage, and pre-term delivery |
|
|
What restricts the passage of thyroid hormones across the placenta?
|
Large amounts of deiodinase enzyme in placenta restrict transplacental passage
|
|
|
Where does the fetus get its thyroid hormones?
|
- Fetal thyroid gland becomes active late in 1st trimester
- Function is dependent on supply of iodine from mother - Maternal thyroid hormones do not significantly pass to fetus because of deiodinase enzyme in placenta |
|
|
What are normal levels of thyroid hormone, during fetal development, critical for? Effects of deficiency?
|
- Neuronal migration and myelination of fetal brain
- Deficiency has adverse effects on neurocognitive functions and may result in severe mental retardation and other neuro symptoms (eg, deaf-mutism) |
|
|
What is the term for excess production of and exposure to thyroid hormones?
|
Thyrotoxicosis
|
|
|
What causes hyperthyroidism in pregnancy? Implications?
|
- Thyrotoxicosis caused by hyper-functioning thyroid gland
- Can increase risk of spontaneous abortion, premature labor, low birth weight, still-birth, pre-eclampsia, and heart failure |
|
|
What is the most common cause of overt hyper-thyroidism? Characteristics?
|
Grave's Disease
- Suppressed TSH and elevated T3 and T4 - Caused by circulating thyroid stimulating IgG auto-antibodies |
|
|
What are the effects of Grave's Disease during pregnancy?
|
- Thyroid stimulating IgG auto-antibodies can cross the placenta and lead to fetal thyroid dysfunction
- Circulating Abs may remain even after surgical or radioactive ablation therapy of thyroid |
|
|
When does Gestational Hyper-Thyroidism occur? How common?
|
- Transient form, limited to first half of pregnancy
- Occurs in 1-3% of pregnancies |
|
|
What is Gestational Hyperthyroidism associated with?
|
- Increased production or increased thyroid-stimulating activity of hCG (as occurs in twin pregnancies)
- Genetic variations in TSH receptors have been described that are more responsive to hCG - Can also occur in hydatiform mole, a tumor of trophoblastic cells |
|
|
What can cause increased production of hCG, leading to gestational hyperthyroidism?
|
Twin pregnancies
|
|
|
What can cause increased thyroid-stimulating activity of hCG, leading to gestational hyperthyroidism?
|
Genetic variations in TSH receptors that are more responsive to hCG
|
|
|
What are the symptoms of gestational hyperthyroidism?
|
- Nausea
- Vomiting - Weight loss - Syndrome of hyperemesis gravidarum observed in 0.05 - 1% of pregnancies |
|
|
How do you manage gestational hyperthyroidism?
|
Self-limiting, so best to manage with supportive treatment like IV fluids, electrolyte replacement, and anti-emetics
|
|
|
What medical emergency is characterized by acute exacerbation of hyperthyroidism?
|
Thyroid Storm
|
|
|
How common is thyroid storm during pregnancy?
|
Only affects 1% of pregnant women with thyrotoxicosis (1-3% of total pregnancies)
|
|
|
What are the symptoms of Thyroid Storm?
|
- Fever
- Tachycardia - Altered mental state (restlessness, seizures, confusion) - Diarrhea - Vomiting - Cardiac arrhythmia |
|
|
What can cause Thyroid Storm?
|
May be associated with inciting event, such as infection, labor, hypoglycemia, or diabetic ketoacidosis
|
|
|
How do you confirm a diagnosis of Thyroid Storm? Implications?
|
Serum TSH and T3/T4 levels, BUT:
- Expedited treatment is required to avoid severe consequences like shock, coma, or death - Treatment should not be withheld pending test results - Treatment initiated immediately to reduce synthesis and release of thyroid hormones, to block peripheral action of thyroid hormones, support physiological functions, and to identify and treat precipitating events |
|
|
What can happen to the pituitary gland during pregnancy?
|
Increase in volume and shape change (up to 30% increase) d/t proliferation of lactotropes (cells that secrete prolactin)
|
|
|
What can happen to healthy pregnant women that have an increased volume and size change of their pituitary?
|
Temporary loss of vision in outer halves of R and L fields (bitemporal hemianopia)
|
|
|
What happens to the anterior pituitary during pregnancy?
|
Cellular composition changes:
- Lactotropes (cells secreting Prolactin) increase from 20% to 60% of all cells by 3rd trimester |
|
|
What are the implications of the lactotropes increasing from 20% to 60% of the total anterior pituitary mass during pregnancy? How is prolactin different?
|
- Lactotropes release Prolactin → Hyperprolactinemia
- Increased production of non-glycosylated isoform of Prolactin, which is more active than glycosylated isoform present in non-pregnant state |
|
|
What happens to prolactin levels in women after giving birth?
|
- Prolactin concentrations in non-lactating women decrease to pre-pregnancy levels within 3 months, but stay higher for a longer period in lactating women
- Serum prolactin conc. is significantly lower in women who have given birth, suggesting a permanent suppression of prolactin secretion following pregnancy |
|
|
What is observed in women exposed to obstetric complications of severe hemorrhage and hypotension at delivery? Where is it more common?
|
Sheehan Syndrome
- More common in areas where deliveries are not performed in healthcare facilities by skilled attendants |
|
|
What happen sin Sheehan Syndrome?
|
- Pituitary and hypothalamus are particularly susceptible to ischemia caused by obstetric hemorrhage and hypotension
- Ischemic injury leads to a loss of hormone reserve which is manifested in failure of lactation, failure of hair growth over areas shaved for delivery, poor wound healing after C-section, and generalized weakness |
|
|
Why are the pituitary and hypothalamus particularly susceptible to ischemia caused by obstetric hemorrhage and hypotension?
|
Pregnancy-related increase in size of pituitary and low-flow, low-pressure nature of the portal circulation
|
|
|
What are the manifestations of ischemic injury in Sheehan Syndrome?
|
Loss of hormone reserve, which is manifested in:
- Failure of lactation - Failure of hair growth over areas shaved for delivery - Poor wound healing after C-section - Generalized weakness |
|
|
What can increase the risk of adult-onset cardiovascular and metabolic disorders in a fetus?
|
- Metabolic or other perturbations of intrauterine environment during fetal development produce permanent changes in fetal structure, function and metabolism
- Some of these are epigenetic changes that alter gene expression and affect biological systems that regulate body weight - Reduced or altered functional capacity develop under adverse in utero conditions |
|
|
What is the definition of Pregnancy-related Maternal Death?
|
WHO:
- Death of a woman while pregnant or within 42 days of termination of pregnancy - Irrespective of the duration and site of the pregnancy, from any cause related to or aggravated by the pregnancy or its management but not from accidental or incidental causes - This definition does not include pregnancy-related maternal deaths that may occur after 42 days of termination of pregnancy CDC: - Death of a woman while pregnant or within 1 year of pregnancy termination from any cause related to or aggravated by the pregnancy or its management, but not from accidental or incidental causes |
|
|
What is the term for the deaths that result directly from pregnancy, labor, delivery, post-partum conditions, or from obstetric complications of pregnancy, from interventions, omissions and incorrect treatments? How common?
|
Direct Obstetric Death (most common cause of maternal death, 75-80%)
|
|
|
What are the leading causes of Direct Obstetric Death in lower or middle income countries?
|
- Hemorrhage
- Sepsis - Hypertensive disorders (pre-eclampsia and eclampsia) (higher income countries are prepared to prevent and treat these) |
|
|
What are the leading causes of Direct Obstetric Death in higher income countries?
|
Thromboembolic events
|
|
|
What is the term for death due to a pre-existing disease (eg, diabetes, cardiac disease, malaria, HIV, TB) or a new disease that is aggravated by the physiological effects of pregnancy? How common?
|
Indirect Obstetric Death (accounts for 20-25% of all maternal deaths)
|
|
|
What is the most commonly used measure of maternal mortality? How is it calculated?
|
Maternal Mortality Ratio (MMR)
- Number of maternal deaths in a population during a given time period per 100,000 live births during same period - Deos not include miscarriages and abortions |
|
|
What is the Maternal Mortality RATE (MMRate)? How is it calculated?
|
- What is the term for the number of maternal deaths in a given period per 100,000 women of reproductive age (15-49 years) during same period
- Does not take into account frequency of pregnancy or birth in women of child-bearing age in a population |
|
|
How does the Maternal Mortality Ratio (MMR) compare in developed to developing countries? MMR in USA?
|
- Developing countries have 15 times higher MMR than developed regions
- USA MMR is 15.8, it has declined dramatically over last century, but trend is reversing in last 3 decades |
|
|
What may be contributing to the increasing MMR in the USA in the last 3 decades?
|
Increase in the number of pregnant women with chronic health conditions, such as HTN and diabetes
|
|
|
What demographic characteristics are risk factors for MMR in the US?
|
*MMR among black women is ~3 times the MMR observed among white women
- Increasing education is protective - Prenatal care lowers the risk of maternal death - Risk is higher in older women, especially over 39 years of age (6x higher than women 15-19 years, and 10x higher for African Americans) |
|
|
What are the most common causes of maternal death world-wide?
|
- Hemorrhage
- Sepsis - HIV/AIDS - Anemia - Obstructed labor |
|
|
What is the most common cause of maternal death in African and Asia? Developed world?
|
- Africa and Asia: hemorrhage (31-34%)
- Developed world: 13% |
|
|
What are the most common causes of maternal death in the US?
|
- Cardiovascular disease (15%)
- Infection / sepsis (14%) - Non-cardiovascular disease (eg, infectious, respiratory, GI, endocrine, hematologic) (12%) - Cardiomyopathy (12%) - Hemorrhage (11%) - HTN disorders (10%) - Thrombotic pulmonary embolism (9%) - Cerebrovascular accident / stroke (6%) - Amniotic fluid embolism (5%) - Anesthesia complication (<1%) - Unknown (5%) |
|
|
What is the definition of a live birth?
|
- Complete expulsion or extraction fo the fetus at any stage of development
- Afterwords infant breathes and has a beating heart, pulsation of umbilical cord, or definite movement of voluntary muscles regardless of whether the umbilical cord has been cut, and regardless of whether the expulsion or extraction occurs as a natural or induced labor, cesarean section, or induced abortion - Transient cardiac contractions and fleeting respiratory efforts or gasps are not considered as signs of life |
|
|
What is the definition of a stillbirth or fetal death?
|
- Death of a fetus prior to expulsion or extraction from the mother
- Fetal death is defined by the absence of signs of life as defined above (breathing, heart beating, pulsating umbilical cord, voluntary muscle movement) - NCHS includes fetal deaths that occur 20 weeks in stillbirths. In some statistics fetal death is divided into early (20 to 27 weeks gestation) or late (28 weeks gestation) |
|
|
What is the recommended information to report regarding a fetal death?
|
Since the gestational age is not always accurately known, ACOG recommends reporting fetal deaths based on birth weight 500g (instead of 20 weeks gestation)
|
|
|
How is the fetal death rate calculated?
|

- Number of fetal deaths at >20 weeks (or >500g) during a year, divided by sum of liver births and stillbirths (= total number of births) during a year
- Expressed per 1000 total births |
|
|
How is the late fetal death rate calculated?
|
- Number of fetal deaths at >28 weeks during a year, divided by sum of liver births and stillbirths (= total number of births) during a year
- Expressed per 1000 total births (Differs from fetal mortality rate by time, fetal mortality rate is based on 20 weeks) |
|
|
What is the definition of a Neonatal Death?
What is the definition of an Early Neonatal Death? What is the definition of a Late Neonatal Death? |
- Neonatal Death: death of a newborn before 28 days of age
- Early: within first 7 days of birth - Late: between 8 and 27 dyas of age |
|
|
How is the Neonatal Mortality Rate calculated?
|
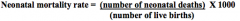
Number of neonatal deaths (before 28 days of age) during a year divided by the number of live births in that year, expressed per 1000 live births
|
|
|
What is the definition of an infant death?
|
Death of a baby before his or her first birthday
|
|
|
How is the Infant Mortality Rate calculated?
|
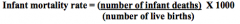
Number of infant deaths (less than 1 year of age) divided by the number of live births reported during same year, expressed per 1000 live births
|
|
|
Where are the highest rates of Infant Mortality Rate? Lowest? US?
|
- Regions in Africa: as high as 100
- Sweden, Norway, Singapore: ~2 - US: ~6 But overall there is a trend of decreasing IMG in the last 20 years |
|
|
What are the common causes of infant mortality world-wide?
|
- Pre-term birth
- Birth asphyxia (lack of breathing at birth) - Infections during neonatal period - Post-neonatal period infections: pneumonia, diarrhea, malaria, measles, HIV/AIDS |
|
|
What are the racial disparities in Infant Mortality Rate (IMR) in the US?
|
A black infant is ~2.3x more likely to die during the first year of life as compared to a white infant (WI has one of greatest disparities)
|
|
|
What are the common causes of infant mortality in the US?
|
- Congenital malformations
- Short gestation and/or low birth weight - SIDS - Maternal complications - Unintentional injuries |
|
|
What is the term for a fetal death in the first trimester?
|
Spontaneous abortion (aka miscarriage)
|
|
|
What is the definition of a spontaneous abortion (eg, miscarriage)?
|
- Fetal death in the first trimester
- Loss of embryo/fetus weight 500g or less, before 20th week of gestation |
|
|
When do you use the term embryo vs fetus?
|
- Embryo: <10 weeks of gestation
- Fetus: >10 weeks of gestation |
|
|
What are the most common causes of fetal death during the FIRST TRIMESTER?
|
- Chromosomal abnormalities
- Genetic abnormalities - Congenital anomalies - Trauma - Uterine anomalies - Acute maternal infection - Unexplained |
|
|
What is the most common cause of fetal death in the first trimester (spontaneous abortion / miscarriage)?
|
Chromosomal abnormalities:
- Account for ~50% of all miscarriages - Mostly aneuploidies - Most arise de novo - Autosomal Trisomy 16 is most common and always lethal |
|
|
What causes congenital anomalies responsible for death in the first trimester?
|
- Chormosomal (mostly aneuploidies) or genetic anomalies (eg, small deletions, duplications, and point mutations in genes)
- Exposure to teratogens (eg, poorly controlled hyperglycemia, medications like isotretinoin, physical stresses like fever, and environmental chemicals like mercury |
|
|
What causes of trauma may cause fetal death in the first trimester?
|
Invasive procedures, including:
- Chorionic villus sampling - Amniocentesis |
|
|
What uterine anomalies can cause fetal death in the first trimester?
|
Interfere with poor implantation and growth, resulting in pregnancy loss:
- Uterine septum (uterine cavity is partitioned by a septum) - Submucosal leiomyomas (benign tumor of uterus arising from smooth muscle |
|
|
What acute maternal infections can cause fetal death in the first trimester?
|
May result in fetal or placental infection and fetal loss:
- CMV - Rubella - Toxoplasma gondii |
|
|
What are the causes of fetal death during the SECOND TRIMESTER?
|
- Cervical insufficiency
- Preterm premature rupture of amniotic membranes - Maternal infection |
|
|
What happens in cervical insufficiency? When does this cause loss of pregnancy? Risk factors?
|
- Occurs during second trimester
- Painless cervical dilation, leading to pregnancy loss - Risk factors: collagen abnormalities, uterine anomalies, prior obstetric trauma or mechanical dilation |
|
|
What happens in preterm premature rupture of amniotic membranes? When does this cause loss of pregnancy?
|
- Occurs during second and third trimesters
- Leads to chorioamnionitis and preterm labor |
|
|
What are the causes of fetal death during the THIRD TRIMESTER?
|
- Preterm premature rupture of amniotic membranes
- Preterm labor - Placental abruption - Umbilical cord compression by fetal head - Intrauterine infection - Placental dysfunction and intrauterine growth restriction (IUGR) - Hemorrhage at uterine/placenta interface - HTN disorders or other medical conditions - Postmaturity |
|
|
What does post-maturity mean? Risks?
|
- A baby that has not yet been born after 42 weeks of gestation, two weeks beyond the normal 40
- Post-mature births can carry risks for both the mother and the infant, including fetal malnutrition - After the 42nd week of gestation, the placenta, which supplies the baby with nutrients and oxygen from the mother, starts aging and will eventually fail - If the fetus passes fecal matter, which is not typical until after birth, and the child breathes it in, then the baby could become sick with pneumonia - Post-term pregnancy may be a reason to induce labor |
|
|
What can cause bleeding in the third trimester of pregnancy?
|
- Effacement (thinning) or dilation of the cervix
- Preterm labor - Placenta previa - Placenta abruption - Vasa Previa - Pathologies of the cervix |
|
|
When does effacement (thinning) or dilation of the cervix occur? What can it cause?
|
- Occurs during 3rd trimester, usually preceding labor by up to 72 hours
- Accompanied small amount of blood with mucus discharge |
|
|
What is the term for a placenta located over or near the internal cervical os?
|
Placenta Previa
|
|
|
What can Placenta Previa cause? When?
|
- Placenta located over or near internal cervical os
- Should be suspected in any woman who presents with vaginal bleeding in the second half of pregnancy - Usually painless, vaginal bleeding without contractions |
|
|
What should you do to determine if your patients cause of bleeding in the second half of pregnancy is caused by Placenta Previa?
|
Ultrasound to confirm placental location prior to any digital exam
|
|
|
How does Placenta Abruption present? Cause?
|
- Presents with vaginal bleeding, uterine tenderness, and contractions, with or without non-reassuring fetal heart tones
- Occurs secondary to placental separation d/t hemorrhage into the decidual basalis |
|
|
What is the difference between the symptoms of Placenta Previa and Placenta Abruption?
|
- Placenta Previa: painless vaginal bleeding, without contractions
- Placental Abruption: vaginal bleeding, uterine tenderness, and contractions |
|
|
What can cause uterine tenderness in Placenta Abruption?
|
Extravasation of blood into the myometrium through the serosa
|
|
|
What kind of diagnosis is Placental Abruption? Why?
|
Diagnosis of exclusion
- Usually cannot be visualized on US exam - Findings of subchorionic hematoma or placenta that covers the internal os support the diagnosis |
|
|
What are the risk factors for Placental Abruption?
|
- History of prior placental abruption
- Trauma - Premature rupture of amniotic membranes - Hypertension - Smoking and cocaine use |
|
|
What is the term for bleeding from the umbilical cord? Implications?
|
Vasa Previa
- Results in loss of fetal blood - Can lead to fetal death |
|
|
What pathologies of the cervix, vagina, or uterus can lead to bleeding in the third trimester?
|
- Cervical polyps
- Inflammation - Infection of cervix, vagina, or uterus - Trauma - Cancer |
|
|
What is the most preventable cause of maternal mortality as well as the leading cause of admission into the ICU?
|
Post-partum Hemorrhage (PPH)
|
|
|
What is the definition of Post-partum Hemorrhage (PPH)?
|
- Blood loss >500 mL after vaginal birth
- Blood loss >1000 mL after cesarean delivery - Makes the patient symptomatic (lightheaded, dizzy, weak, palpitations, diaphoresis, pallor, restlessness, syncope) or signs of hypovolemia (hypotension, tachycardia, oliguria, or low O2 sats) |
|
|
What are the two categories of post-partum hemorrhage (PPH)?
|
- Primary: within 24 hours of delivery
- Secondary: between 24 hours and 12 weeks of delivery |
|
|
What controls uterine bleeding after delivery?
|
- Contraction of myometrium (constricts blood vessels of placental bed)
- Local decidual hemostatic factors (tissue factor, type-1 plasminogen activator inhibitor) - Systematic coagulation factors (circulating clotting factors and platelets) |
|
|
What are the common causes of post-partum hemorrhage (PPH)?
|
- Uterine atony
- Trauma-related bleeding - Coagulation defects secondary to other disorders - Congenital bleeding diatheses |
|
|
What is the most common cause of Post-partum Hemorrhage (PPH)? What does this mean?
|
Uterine Atony
|
|
|
What is Uterine Atony? What can it cause?
|
- Lack of effective contraction of uterus after delivery
- Most common cause of PPH |
|
|
What is the term for a lack of effective contraction of the uterus after delivery? Risk factors?
|
Uterine Atony, risk factors:
- Over distention of uterus (secondary to multiple gestation, polyhydramnios, macrosomia) - Uterine infection - Drugs that relax uterus - Prolonged labor - Uterine inversion - Retained placenta or placental fragments - Can also occur in the absence of any of these risk factors |
|
|
What can cause trauma-related post-partum hemorrhage (PPH)?
|
- Lacerations of the perineum, vagina, cervix, or uterus (usually after instrumental delivery)
- Incisions (eg, episiotomy, hysterotomy) - Uterine rupture |
|
|
What coagulation defects secondary to other disorders can cause post-partum hemorrhage (PPH)?
|
- Severe pre-eclampsia
- HELLP syndrome - Placental abruption - Fetal demise - Amniotic fluid embolism - Sepsis - Surgical site bleeding |
|
|
What are congenital bleeding disorders related to that can cause post-partum hemorrhage (PPH)?
|
Congenital bleeding diatheses related to hemodilution, failure of liver synthetic function or disseminated intravascular coagulation
|
|
|
What are the risk factors for post-partum hemorrhage (PPH)?
|
In decreasing order of frequency:
- Retained placenta - Prolonged labor or failure to progress during second stage of labor - Placenta accreta - Lacerations - Instrumental delivery - Macrosomia - Hypertensive disorders - Induction of labor - Augmentation of labor with oxytocin - Placenta previa - History of previous PPH - Obesity - High parity - Precipitous labor - Prolonged first stage of labor > 24 hours - Uterine over-distention - Uterine infection - Pre-eclampsia - Factor XI deficiency - Hemophilia carriers |
|
|
What is the term for a fetus that has not reached its growth potential because of genetic or environmental factors?
|
Intrauterine Growth Restriction (IUGR)
|
|
|
What may cause Intrauterine Growth Restriction (IUGR)?
|
Fetal, Placental, or Maternal Factors (or a combination of these)
|
|
|
What are the Fetal causes of Intrauterine Growth Restriction (IUGR)?
|
- Genetic (eg, aneuploidy)
- Major congenital anomalies - Multiple gestation - Infection |
|
|
What negative effect can multiple gestations have on the development of the fetuses?
|
Cause of Intrauterine Growth Restriction (IUGR)
- Lower weight of fetuses in multiple gestations is thought to be d/t inability of environment to meet nutritional needs of multiple fetuses - Also common complications in multiple gestation include maternal under-nutrition, pre-eclampsia, congenital anomalies, twin-twin transfusion) |
|
|
What infections can affect the fetus and cause Intrauterine Growth Restriction (IUGR)?
|
Overall rare, but occur particularly with viruses and parasites:
- Rubella - Toxoplasmosis - CMV - Syphilis - Herpes simplex |
|
|
What are the Placental causes of Intrauterine Growth Restriction (IUGR)?
|
- Pre-Eclampsia
- Abruption - Abnormal vascular connections in the placenta - Diffuse choronic villitis (inflam. of placental villi) - Placental structural anomalies - Confined placental mosaicism |
|
|
What placental structural anomalies can cause Intrauterine Growth Restriction (IUGR)?
|
- Single umbilical artery (instead of two)
- Velamentous umbilical cord insertion (abnormal insertion of cord into placenta making vein more prone to rupture and cause vasa previa) - Marginal cord insertion |
|
|
What does confined placental mosaicism refer to?
|
Chromosomal mosaicism, usually involving a trisomy found in the placenta but not in the fetus
|
|
|
What are the Maternal causes of Intrauterine Growth Restriction (IUGR)?
|
Disorders that reduce uteroplacental blood flow d/t abnormal development, acquired obstruction, or disruption of uteroplacental vasculature:
- HTN - Renal insufficiency - Diabetes - Collagen Vascular Disease - Systemic Lupus Erythematosus - Anti-Phospholipid Syndrome - Many more |
|
|
What is the term for fetal growth beyond a specific threshold? What size meets this definition?
|
Macrosomia: >5000g without diabetes or >4500g with diabetes
|
|
|
What is the significance of a fetus weighing more than 4500g?
|
Macrosomia - they are at increased risk for morbidity above this weight
|
|
|
What are the most important risk factors for Macrosomia?
|
- High BMI
- Multiparity - Advanced maternal age - Maternal diabetes / gestational diabetes - Post-term pregnancy - Male infant - Previous macrosomic infant - Excessive weight gain in pregnancy - Hispanic or African-American ethnicity - Maternal BW >4000g - Fetal genetic abnormalities |
|
|
What fetal genetic abnormalities are associated with Macrosomia (BW >4500g)?
|
- Pallister-Killian (rare mosaicism of isochromosome 12p)
- Beckwith-Widemann (abnormality in chromosome 11 resulting in overgrowth and abnormal growth) |
|
|
How often should prenatal visits occur?
|
- Every 4 weeks until 28 weeks
- Every 2 weeks until 36 weeks - Weekly until delivery |
|
|
What are the routine assessments done at every prenatal visit?
|
- BP
- Weight - Urine dipstick for protein - Measurement of uterine size or fundal height to assess for fetal growth - Documentation of fetal cardiac activity - Assessment of maternal perception of fetal activity (in 2nd and 3rd trimesters) - Assessment of fetal presentation (3rd trimester) |
|
|
When should a woman be induced normally?
|
If not delivered by 40 weeks gestation
|
|
|
What is the first trimester? When should your first OB visit be ideally?
|
- First trimester = first 12 weeks of pregnancy
- Ideally initiate first visit by week 10 |
|
|
What are the goals of the first OB visit?
|
- Estimate gestational age or estimated date of delivery (EDD)
- Obtain detailed patient history - Create a problem list - Physical exam - Routine prenatal labs - Offer genetic screening such as first trimester screen - Patient education |
|
|
How do you estimate the date of delivery?
|
Calculate from menstrual history in woman with 28-day cycles by:
- Add 7 days - Subtract 3 months |
|
|
If a patients last menstrual period (LMP) was January 1, 2013, what is the estimated date of delivery?
|
Add 7 days
Subtract 3 months From Jan 1 → Oct. 8 |
|
|
What patient history should you get from a patient on their first OB visit?
|
- Personal and demographic information
- Past OB history - Personal and family medical history (eg, chronic HTN or DM) - Past surgical history - Genetic history (evaluation of inherited disorders such as hemoglobinopathies, cystic fibrosis, fragile X, etc) - Menstrual and gyencologic history - Current pregnancy - Psychosocial information (eg, domestic violence, barriers to care) |
|
|
What physical exam techniques should you use to evaluate your patient on her first OB visit?
|
- Baseline BP
- Weight, height, BMI - Pelvic exam (assess uterine size, shape, and position) - Auscultation of fetal heart sounds using handheld Doppler |
|
|
What are the routine prenatal labs you should get at the first OB visit?
|
- Urine pregnancy test
- Blood type and antibody screen - CBC - Pap smear - Rubella and varicella immunity - Urine protein and culture - Syphilis testing - Hepatitis B antigen testing - Chlamydia and gonorrhea testing - HIV testing - In higher risk women: TSH, HbA1c |
|
|
When can the first trimester screen for genetic concerns be administered?
|
Between 11w0d and 13w6d
|
|
|
What are the components of the First Trimester Screen?
|
- Ultrasound with measurement of nuchal translucency to assess for fetal aneuploidy (eg, Down Syndrome, Trisomy 13, Trisomy 18, sex chromosome aneuploidy)
- Women with Advanced Maternal Age or fetuses with congenital anomalies on US may qualify for cell free fetal DNA testing |
|
|
What patient education should be provided to a mother at her first OB appointment?
|
- Discuss their responsibilities and expected course of pregnancy and delivery
- Discuss seat belt use, vitamins, nutrition, and weight gain - Avoid alcohol, cigarettes, illicit drugs - Infection precautions (flu shot and making sure TDAP is up to date) - Avoid cat litter - Work and exercise - Oral health, avoidance of hottubs, and saunas - Medications to avoid, caffeine intake, methylmercury in fish |
|
|
What is the time period of the second trimester?
|
>12 weeks gestation to <26 weeks gestation
|
|
|
What do you have ongoing measurement of in the second trimester OB visits?
|
- Maternal BP and weight
- Urine dipstick for protein - Document fetal cardiac activity - Maternal perception of fetal activity |
|
|
What is the MSAFP serum test for? When can it be administered?
|
- Screen for neural tube defects
- Between 15w0d to 22w0d |
|
|
What is the Quad Screen for? When can it be administered?
|
- Screens for aneuploidy (eg, Trisomies 21, 13, 18, and sex chromosome aneuploidy)
- 15w0d to 22w0d if first trimester screen window is missed |
|
|
What is the Fetal Anatomy Survey for? When can it be administered?
|
- Screen for structural anomalies
- Checks cervical length - 18w0d to 22w0d |
|
|
When is the third trimester? What tests need to be done during this time?
|
- 26 weeks to 40 weeks gestation
- Glucola screen for gestational diabetes - Re-check hemoglobin or hematocrit early on for anemia - STD screening in women diagnosed w/ STD earlier in pregnancy, continue to have risk factors, or have acquired a new risk factor during pregnancy - Group B Strep screening at 36 weeks |
|
|
What is the function of the Glucola screen? When is it done?
|
- Screen for gestational diabetes
- Between 24 to 28 weeks gestation - Should be performed in 1st trimester in women with significant risk factors such as BMI>30 |
|
|
What should you do for patients that are Rh negative? When?
|
- Repeat antibody screen
- Receive Rhogam around 28 weeks (3rd trimester) |
|
|
What is the definition of anemia throughout pregnancy?
|
- 1st and 3rd trimesters: Hemoglobin < 11g/dL or Hematocrit <33%
- 2nd trimester: Hemoglobin < 10.5g/dL or Hematocrit <32% |
|
|
Under what circumstances should you do STD screening during the third trimester?
|
- Women who were diagnosed with STD earlier in pregnancy
- Women who continue to have risk factors - Women who have acquired new risk factor during pregnancy - CDC recommends re-testing all women <25 years of age for Chlamydia in 3rd trimester - Some areas have mandatory testing for HIV and syphilis |
|
|
When should you do Group B Strep screening?
|
At 36 weeks
|
|
|
What are the risks of low or inadequate weight gain during pregnancy?
|
Increased risk of preterm delivery and low birth weight, regardless of pre-pregnancy BMI levels
|
|
|
How does obesity with GDM affect the risk of your pregnancy compared to a normal weight woman with GDM?
|
Obese women with GDM have higher risk of adverse perinatal outcomes
|
|
|
Is caloric restriction okay for obese pregnant women with GDM?
|
Yes (~70% DRI), can result in a considerable slowing of maternal weight gain, without causing maternal or fetal compromise and/or ketonuria
|
|
|
What is the requirement of carbohydrates per day in pregnant women (including those with GDM)? Why?
|
- Minimum of 175g of carbohydrate/day
- Important for fetal brain development and to prevent ketosis - In women with GDM, carbohydrate intake should be <45% of energy to prevent hyperglycemia |
|
|
What is associated with increased post-prandial blood glucose levels?
|
Increased incidence of large-for-gestational age infants and rate of C-sections
|
|
|
What are the caloric requirements for a lactating woman? Suggestions?
|
- Energy cost of exclusive breast-feeding from birth to six months is ~500 kcal/day
- This is subsidized by mobilization of tissue stores (~170 kcal/day) * Therefore, recommended dietary allowance for energy is ~330 kcal/day more than a non-pregnant, non-lactating woman |
|
|
Under what nutritional circumstances would breast milk volume decline?
|
Only in extreme energy deprivation (<1500 kcal/day)
|
|
|
What are the nutritional (vitamin) requirements for a lactating woman? Suggestions?
|
- Fat and water soluble vitamins are secreted into milk
- Increased requirements for most vitamins - Balanced diet of protein, fatty acids, fat-soluble vitamins, Vitamin D supplements (if sufficient), and water-soluble vitamins are needed - Diet can be supplemented with a multi-vitamin |
|
|
What are the protein requirements for a lactating woman?
|
- 25g/day for first six months
- Fish and shellfish contain high-quality protein and other essential nutrients including omega-3 fatty acids (important for brain development) - However, fish and shellfish may contain mercury; eat up to 2 average servings (or 12 oz / week) of fish and shellfish that contain low conc. of mercury (shrimp, canned light tuna, salmon, pollock, catfish) |
|
|
How much milk is produced per day in an exclusively breastfeeding woman?
|
- Healthy, exclusively breastfeeding women make 750-800 cc/day when milk supply is fully established
- Can average 450-1200 cc/day - Milk volume is low on the first two days post-partum, increases on days 3-4, and gradually increases to full lactation |
|
|
What determines milk production?
|
* Mostly by infant demand
- Stress, anxiety, fatigue, illness, incomplete emptying, infrequent milk expression, and smoking can decrease production |
|
|
When should you screen for post-partum depression?
|
During prenatal care and during postpartum period:
- 28 week prenatal visit - Prior to discharge from hospital - 6 week postpartum visit - Women who report depressive symptoms without suicidal ideation or major functional impairment are re-evaluated within 1 month to determine the state of depression |
|
|
What are the indications for genetic testing and counseling?
|
- Advanced maternal age (>35y)
- Advanced paternal age (>40-45y) - History of recurrent pregnancy loss, stillbirth, or infant death - Parental carriers of a genetic disorder or chromosome abnormality - Family history involving a birth defect, mental retardation, chromosome abnormality, or genetic disorder - Maternal teratogen exposure(s) during pregnancy - Ethnic background associated with increased prevalence of a heritable disorder - Consanguinity - Pre-implantation genetic diagnosis (PGD) for evaluation of embryos prior to implantation |
|
|
At what maternal age is genetic testing and counseling indicated?
|
Maternal age 35 or older at estimated date of delivery (EDD)
|
|
|
What is the risk for a major aneuploidy at live birth depending on maternal age?
|
- Mom >35, with singleton pregnancy risk is 1:385
- Mom >33, with twin pregnancy risk is 1:176 for at least one twin |
|
|
What is the risk associated with advanced paternal age (40-45) that you would check for with genetic testing and counseling?
|
- Risk of major aneuploidy is not increased
- There is an association with de novo single gene mutations (diseases) |
|
|
What are the methods for genetic testing of a fetus?
|
Performed before birth using cells derived from the placenta, amniotic fluid, cell-free fetal DNA in maternal blood, fetal blood, or fetal tissue
|
|
|
What is PGD genetic testing? When is it used?
|
Pre-Implantation Genetic Diagnosis (PGD):
- Cells derived from in vitro fertilized embryos (pre-implanted blastomere) can be used to screen for genetic defects and select unaffected embryos |
|
|
When is a first trimester genetic screen indicated?
|
- Offered as a ROUTINE test for risk assessment of fetal Down syndrome, trisomy 13, and trisomy 18
- Indicated for all pregnant patients regardless of fetal aneuploidy risk |
|
|
When is the optimal time for a first trimester genetic screen?
|
Between 11w0d and 13w6d
|
|
|
What specific tests are done during a First Trimester Screen (between 11w0d and 13w6d)?
|
- Fetal US for nucal translucency (NT) and nasal bone measurement
- Serum screening for Plasma-Associated Protein A (PAPP-A) and free β-human Chorionic Gonadotropin (β-hCG) |
|
|
What are the signs during a First Trimester Screen that would indicate Down Syndrome?
|
- Nuchal Translucency (NT) is higher
- β-human Chorionic Gonadotropin (β-hCG) is higher - Plasma-Associated Protein A (PAPP-A) is lower |
|
|
How good is the detect rate in the First Trimester Screen for Down Syndrome and Trisomies 13 and 18?
|
- 91% accurate for Down Syndrome w/ 5% false positive rate (FPR)
- Improves to 95% with 2% FPR when nasal bone measurement is included 95% for Trisomy 18 and 13 with FPR <1%, although limited data for Trisomy 13 |
|
|
What is the indication for a cell-free fetal (cff) DNA / non-invasive prenatal screening (NIPS)?
|
Offereed for pregnancies at INCREASED RISK for fetal Down syndrome, trisomies 13 and 18, or sex chromosome abnormalities (ie, Turner syndrome)
High risk factors include: - Advanced maternal age - Previous pregnancy or positive family history of common aneuploidy - Abnormal fetal US or screening blood test suggestive of a chromosome abnormality Other indications: - Fetal Rh genotyping - Prenatal paternity testing - Evaluation of other trisomies and chromosome deletions / duplications |
|
|
When is the optimal time to do a cell-free fetal (cff) DNA / non-invasive prenatal screening (NIPS)?
|
Start at 10w0d until EDD
|
|
|
What is tested during the cell-free fetal (cff) DNA / non-invasive prenatal screening (NIPS)?
|
Utilizes short DNA fragments (50-300 base pairs) known as cell-free fetal DNA (cffDNA) in maternal serum
|
|
|
What are the detection rates for the cell-free fetal (cff) DNA / non-invasive prenatal screening (NIPS)?
|
- 99.2-99.9% for Down Syndrome
- 97-99.8% for Trisomy 18 - 80-99% for Trisomy 13 - 99.1-99.9% for Y chromosome detection - 94-96% for monosomy X (Turner syndrome) * Specificity is >98-99% based on screening high risk cases |
|
|
When is the Maternal Serum Quad Screen indicated?
|
Offered as a ROUTINE test for risk assessment of
- Fetal Down Syndrome - Trisomy 18 - Neural tube defects (spina bifida, anencephaly) - Abdominal wall defects (gastroschisis) *Indicated in all pregnant patients regardless of fetal aneuploidy risk |
|
|
When is the optimal time to do the Materal Serum Quad Screen?
|
Between 15w0d and 20w6d
|
|
|
What are the test parameters for the Maternal Serum Quad Screen?
|
- Alpha-Fetoprotein (AFP) - produced in fetal liver and yolk sac (decreased in Down Syndrome)
- Human Chorionic Gonadotropin (hCG) - synthesized by placenta and essential for early pregnancy maintenance (increased) - Unconjugated Estriol (uE3) - produced in placenta (decreased in Down Syndrome) - Dimeric Inhibin A (DIA) - synthesized by gonads, corpus luteum, decidua, and placenta (increased in Down Syndrome) |
|
|
What serum Alpha Fetoprotein (AFP) level indicates Down Syndrome in the Maternal Serum Quad Screen? Where is this hormone produced?
|
Decreased in Down Syndrome
- AFP is produced in fetal liver and yolk sac |
|
|
What serum Human Chorionic Gonadotropin (hCG) level indicates Down Syndrome in the Maternal Serum Quad Screen? Where is this hormone produced?
|
Increased in Down Syndrome
- hCG is synthesized by placenta and essential for early pregnancy maintenance |
|
|
What serum Unconjugated Estriol (uE3) level indicates Down Syndrome in the Maternal Serum Quad Screen? Where is this hormone produced?
|
Decreased in Down Syndrome
- uE3 is produced in placenta with weak estrogenic activity |
|
|
What serum Dimeric Inhibin A (DIA) level indicates Down Syndrome in the Maternal Serum Quad Screen? Where is this hormone produced?
|
Increased in Down Syndrome
- Synthesized by gonads, corpus luteum, decidua, and placenta |
|
|
Which of the Maternal Serum Quad Screen serum markers are elevated in Down Syndrome? Decreased?
|
Increased:
- Human Chorionic Gonadotropin (hCG) - Dimeric Inhibin A (DIA Decreased: - Alpha-Fetoprotein (AFP) - Unconjugated Estriol (uE3) |
|
|
What are the detection rates for the four genetic anomalies that are screened in the Maternal Serum Quad Screen?
|
- Down Syndrome: 85%
- Trisomy 18: 60-70% - Neural Tube Defects: 85-90% - Gastroschisis: 85% |
|
|
What are the Prenatal Genetic Diagnostic Tests? How effective are the detection rates for these tests?
|
- Chorionic Villus Sampling (CVS)
- Amniocentesis - Cordocentesis / Percutaneous Umbilical Blood Sampling (PUBS) - All have detection rates >99% for chromosome abnormalities |
|
|
When is the Chorionic Villus Sampling (CVS) test indicated?
|
Prenatal diagnosis of:
- Fetal aneuploidy (indicated for increased risk d/t advanced maternal age, family history, or abnormal first trimester screen) - Other chromosome disorders (ie, deletion) - Single gene disorders - Does not screen for neural tube defects |
|
|
What is the optimal time of the Chorionic Villus Sampling (CVS) test?
|
11w0d to 14w0d
|
|
|
What are the test parameters of the Chorionic Villus Sampling (CVS)?
|
Sample of the placental chorionic villi, obtained by transabdominal or transcervical method for chromosomal or DNA analysis
|
|
|
What are the limitations of the Chorionic Villus Sampling (CVS)?
|
- Pregnancy loss rate with CVS is ~1% above baseline risk of 2-5% at 10-12 weeks gestation
- ~2% risk that CVS identifies confined placental mosaicism (CPM) - CPM can compromise placental function and increase risk for IUGR (intrauterine growth restriction) and perinatal death |
|
|
When is an Amniocentesis indicated?
|
- Prenatal diagnosis of fetal aneuploidy, other chromosomal abnormalities, single gene disorders, and abnormal biochemical levels
- Amniotic fluid AFP analysis and acetyl cholinesterase (AChE) for detection of open neural tube defects and abdominal wall defects - Indicated for pregnancies with increased risk due to advanced maternal age, family history or abnormal screen. |
|
|
When is the optimal time for an Amniocentesis?
|
- 15w0d to 23w0d, but may be performed any time after 15w
- Risk of pregnancy loss after 15-16 weeks gestation, especially after 20-22 weeks |
|
|
What are the test parameters for an Amniocentesis?
|
A sample of amniotic fluid is collected and floating fetal cells are used for culture, chromosomal and DNA analysis
|
|
|
What are the limitations of an Amniocentesis?
|
- Pregnancy loss rate most often is quoted to be 1:300-500 above the baseline risk if done after 15-16 weeks gestation
- Some studies report lower risk, closer to 1:1600 - Risk increases to 3% after 20-22 weeks gestation |
|
|
What is the indication for a Cordocentesis / Percutaneous Umbilical Blood Sampling?
|
- Used as a follow-up when amniocentesis culture fails or yields ambiguous results
- Used when biochemical tests of fetal plasma or blood cells are needed - Used infection need to be confirmed |
|
|
When is the optimal time for Cordocentesis / Percutaneous Umbilical Blood Sampling?
|
Typically at 19-21 weeks
|
|
|
What are the test parameters for Cordocentesis / Percutaneous Umbilical Blood Sampling? Risks?
|
- Sample fetal blood directly from umbilical vein with US guidance or infusion of blood products
- Pregnancy loss rate is 1-2% |
|
|
What ethnic groups should be screened for Cystic Fibrosis?
|
All ethnic groups
|
|
|
What ethnic groups should be screened for Hemoglobinopathies?
|
- African American
- Asian - Latino - Mediterranean |
|
|
What ethnic groups should be screened for Tay-Sachs disease?
|
- French Canadian
- Jews |
|
|
What ethnic groups should be screened for Familial Dysautonomia?
|
Jews
|
|
|
What ethnic groups should be screened for Canavan disease?
|
Jews
|
|
|
What is the method for carrier screening?
|
Genotyping of selected disease-causing mutations within a gene(s)
- A normal or negative carrier screening result can not eliminate an individual's carrier risk, it only reduces the risk - Single gene carrier screening is available in addition to expanded carrier screening panels of up to 100+ inherited conditions |
|
|
What are the symptoms of Fragile X syndrome?
|
- Mild to profound mental retardation
- Behavior abnormalities / autistic tendencies - Macrocephaly and other subtle facial characteristics - Increased risk for premature ovarian failure among females - Increased risk for adult-onset cerebellar ataxia / intention tremors |
|
|
What are the symptoms of Spinal Muscular Atrophy?
|
- Onset from before birth to young adulthood
- Progressive muscle weakness and atrophy - Poor weight gain - Sleep difficulties - Pneumonia - Scoliosis - Joint contractures |
|
|
What disease-causing mutations can be screened for carrier status?
|
- Cystic Fibrosis
- Hemoglobinopathies - Jewish ancestry related diseases (Tay-Sachs, Familial dysautonomia, Canavan disease) - Fragile X syndrome - Spinal muscular atrophy |
|
|
What are the goals of "newborn screening"?
|
Public health program to identify newborns with metabolic and inherited disorders that are threatening to life or long-term health before they become symptomatic
|
|
|
What are some examples of diseases/disorders that are screened for on Newborn Screening?
|
- Endocrine disorders
- Hemoglobinopathies - Cystic fibrosis - Immunodeficiency - Congenital hearing loss - Congenital heart defects |
|
|
What are the benefits of Newborn Screening?
|
Significantly reduces the number of infant deaths, illness, and related disabilities
|
|
|
What kind of tests are done for Newborn Screening?
|
- Radioimmunoassay (T4 for congenital hypothyroidism)
- Liquid chromatography (hemoglobinopathies) - PCR for screening gene mutations - Tandem mass spectrometry (lysosomal storage disorders) - Others |
|
|
How are specimens collected for Newborn Screening?
|
- Heel stick between 24-48 hours of life
- Specimen must be collected before discharge from the hospital of birth - If initial specimen is collected before 24 hours, a repeat specimen is recommended in about 14 days - If newborn is ill, presents with a low birth weight, or requires a transfusion, specifications are available about timing and number of specimen collections |
|
|
Which of the following statements about the human placenta is FALSE?
a) The human placenta is hemochorial b) The chorionic villus is the functional unit of the placenta; it contains an outer syncytiotrophoblast and an inner cytotrophoblast layer, stromal cells and the fetal vascular endothelium, separating fetal circulation from maternal blood c) Maternal blood flow through intervillous space begins around 10 to 14 weeks of gestation d) The placenta is not innervated; it communicates with the mother by directly secreting hormones into maternal circulation e) Maternal tissues communicate with the fetus by directly secreting hormones into fetal circulation |
False: Maternal tissues communicate with the fetus by directly secreting hormones into fetal circulation
|
|
|
What is the functional unit of the placenta? Components?
|
Chorionic villus - contains an outer syncytiotrophoblast and an inner cytotrophoblast layer, stromal cells, and the fetal vascular endothelium, separating fetal circulation from maternal blood
|
|
|
What are the outer and inner layers of the chorionic villus (functional unit of placenta)?
|
- Outer: Syncytiotrophoblast
- Inner: Cytotrophoblast |
|
|
When does maternal blood flow through the intervillous space begin?
|
10-14 weeks gestation
|
|
|
How does the placenta communicate with the mother?
|
Directly secreting hormones into the maternal circulation (it is not innervated)
|
|
|
What are the characteristics of Hemochorial Placentation?
|
- Placental control of maternal physiology
- Maternal blood comes into direct contact with the fetal chorion, may allow for more efficient transfer of nutrients, etc |
|
|
What hormone are released by the placenta?
|
- hCG (human chorionic gonadotropin)
- Progesterones and Estrogens - GnRH (gonadotropin releasing hormone) - hPGH (human placental growth hormone) - hPL (human placental lactogen) - CRH (corticotrophin-releasing hormone) - POMC (proopiomelanocortin) - Leptin - Ghrelin(?) |
|
|
Which hormones are released by the hypothalamus?
|
- Prolactin
- POMC (proopiomelanocortin) --> ACTH --> glucocorticoids |
|
|
All of the following hormones are increased in pregnancy. Several of them are made in the placenta. Which of them is primarily made in maternal endocrine glands?
a) Prolactin (PRL) b) CRH c) Chorionic Gonadotropin (hCG) d) Placental lactogen (hPL) e) Progesterone |
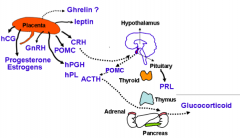
Prolactin
|
|
|
A 25-year old woman gives birth to a 4050 g baby boy at 36 weeks gestation. She says her sister had high blood sugar in pregnancy. The infant has brachycardia and respiratory distress and his serum is sent out for laboratory evaluation. Which of the following abnormalities would you expect to see in the infant?
a) Hypoinsulinemia b) Hyperglycemia c) Hypoglycemia d) Hypercalcemia e) Hypermagnesemia |
Hypoglycemia
Infant has lots of insulin from mom, so once they are born and no longer receiving tons of glucose from mom, they become hypoglycemic d/t hyperinsulinemia |
|
|
What mechanisms are implicated in the pathogenesis of preeclampsia?
|
- Incomplete spiral artery remodeling
- Defective trophoblast differentiation - Placental hypoperfusion - sFlt1 - sEng - AT1 autoantibodies |
|
|
A 32-year old G1P0 woman at 36-week gestation is brought to the emergency department with contractions. Her blood pressure is 180/120 mmHg. Laboratory tests show a platelet count of 6000/microliter and elevated liver enzymes. The patient is treated with an intravenous medication and taken for a cesarean section. Which of the following is most likely an adverse effect of the medication this patient received?
a) Severe mood swings b) Stevens-Johnson syndrome c) Extensor plantar reflex d) Decreased tendon reflexes e) Contraction of the facial muscle when tapped What drug? |
Decreased tendon reflexes (d/t Magnesium Sulfate)
|
|
|
What is the correct definition of maternal mortality ratio (MMR)?
|
Number of maternal deaths in a population per 100,000 live births in the same population during a given period.
|
|
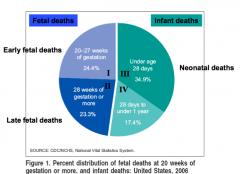
Which of these numbers (I, II, III, and IV) are considered part of "Perinatal Death"?
|
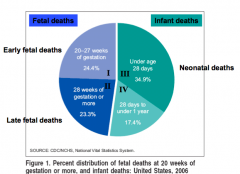
I, II, and III
- I: 20-27 weeks of gestation - II: 28+ weeks of gestation - III: under age 28 days |
|
|
What is the correct definition of neonatal mortality rate?
|
Number of neonatal deaths per 1000 live births
|
|
|
What is the correct definition of perinatal mortality rate?
|
Number of neonatal deaths plus fetal deaths per 1000 total births
|
|
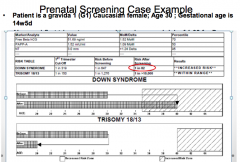
What should be included as part of your genetic risk assessment discussion with this patient?
- 30 year old Caucasian female gravida 1 - Gestational age is 14w5d - Risk after First Trimester Screen is a 1 in 82 chance of Down Syndrome |
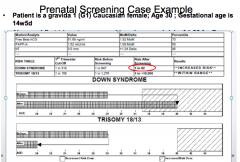
- Discuss the purpose of the first trimester screen and the false-positive rate
- Review and offer additional prenatal testing options using non-invasive prenatal screening (NIPS) or amniocentesis |
|
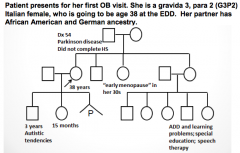
What is the best approach regarding carrier screening recommendations for this patient.
|
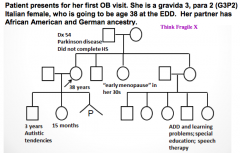
Carrier screening for cystic fibrosis, hemoglobinopathies, and Fragile X syndrome should be offered if it has not been completed in a previous pregnancy
|
|
|
AR is a 35 yo Gravida 2, Para 1 at 8 weeks gestational age by last menstrual period, presenting to her OB with intermittent spotting for the past 2 weeks. A transvaginal ultrasound is performed which demonstrates a gestational sac with a fetal pole, however no cardiac activity is visualized. What is the likely diagnosis?
a) Uterine septum b) Cytomegalovirus infection c) Trisomy 16 d) Alcohol use |
Trisomy 16 (chromosomal anomalies are the most common cause of fetal death in the 1st trimester)
|
|
|
CY is a 24 yo gravida 2, para 1 at 18 weeks gestation. She has a history of a prior loss at 16 weeks gestation. She presents to the emergency room with heavy vaginal bleeding and leaking of fluid. She denies any abdominal pain. What is the most likely etiology?
a) Placental abruption b) Maternal influenza infection c) Preterm labor d) Cervical insufficiency |
Cervical Insufficiency (painless cervical dilation leading to pregnancy loss in 2nd trimester)
Abruption is associated w/ contractions and pain Influenza could lead to infected uterus leading to ruptured membranes, but the most likely cause is cervical insufficiency |
|
|
LW is a 36 yo G1P0 at 22 wga. PMH significant for CHTN on labetalol & end stage renal disease with proteinuria. She presents to L&D with severe range blood pressures (160-180/100-110) and blood work demonstrates AST-80, ALT 84, and Plt 80,000. Bedside sono demonstrates weight <3 percentile and no fetal heart activity. What is the most likely etiology?
a) Placental abruption b) Placental dysfunction c) Umbilical cord compression d) Intrauterine infection |
Placental Dysfunction
(Placental abruption should present with severe bleeding and contractions; Infection is unlikely without a fever; Umbilical cord compression is less likely) |
|
|
KQ is a 33 yo gravida 1, para 0 at 6 weeks gestational age presenting for her first prenatal visit. PMH significant for a BMI of 43 and chronic hypertension. What should be accomplished at this visit?
a) Detailed history b) Physical exam c) Routine prenatal labs d) Early glucola e) All of the above |
All of the above
|
|
|
RK is a 32 yo G2P1 at 37 weeks gestation, presenting for a growth ultrasound for uterine size less than expected for dates. PMH significant for chronic hypertension. An ultrasound demonstrates growth <10 percentile with normal blood flow in the umbilical cord as demonstrated by the umbilical artery Doppler. What is the most likely etiology?
a) Major congenital anomaly b) Placental abruption c) Placental insufficiency d) Chorioamnionitis |
Placental Insufficiency
|
|
|
MM is a 35 yo G9P8 who presents in active labor to L&D. She is found to be 8 cm dilated, and within ten minutes delivers a healthy infant via a normal spontaneous vaginal delivery. Shortly after the delivery of the placenta, she experiences brisk vaginal bleeding. What is the most likely etiology?
a) Cervical lacertion b) Uterine atony c) Placental abruption d) Von Willebrand’s disease Uterine atony more common with multiple gestations. Cervical laceration is possible but with this many births it is unlikely. |
Uterine Atony - more common with multiple gestations (G9P8)
|
|
|
MM, the 35-year old Gravida 9 Para 9 had brisk vaginal bleeding after delivery due to uterine atony. This resulted in an excessive blood loss of over 5 liters. She presents for a follow up visit to her Ob/Gyn 6 months after delivery with complaints of not having a period. She also has not been able to breastfeed secondary to poor milk production. What is the likely diagnosis?
a) Pregnancy b) Postpartum hypopituitarism c) Postpartum hypothyroidism d) Pituitary adenoma |
Postpartum hypopituitarism
|
|
|
Gestational Diabetes:
- Definition - Risk factors - Diagnosis - Management - Fetal outcome - Maternal outcome |
- Definition: onset of abnormal glucose tolerance during pregnancy
- Risk factors: obesity, previous history, family history, African American, Hispanic, Pacific Islander, Asian ethnicity - Diagnosis: glucola screen - Management: proper glycemic control - Fetal outcome: associated with fetal macrosomia, polyhydramnios, neonatal hypoglycemia, risk of birth trauma, long term risk of metabolic disorders - Maternal outcome: future risk of diabetes |
|
|
Gestational Hypertension
- Definition - Diagnosis |
New onset hypertension in pregnancy with systolic pressure >140 mmHg and/or diastolic pressure >90 mmHg measured at least 4 hours apart
|
|
|
Pre-Eclampsia
- Definition - Risk factors - Diagnosis - Management - Fetal outcome - Maternal outcome |
- Definition: severe hypertension with systolic pressure >160mmHg and/or diastolic pressure >110mmHg with proteinuria and/or signs of end organ dysfunction
- Risk factors: pre-existing conditions such as obesity, diabetes, anti-phospholipid syndrome, previous history, family history, primiparity, maternal age <20 or >35 - Diagnosis: clinical, based on HTN and proteinuria and/or evidence of end organ dysfunction - Management: close monitoring and delivery, if disease becomes severe with risk of maternal/fetal morbidity - Fetal outcome: associated with fetal growth restriction, oligohydramnios, and fetal death - Maternal outcome: can advance to eclampsia and maternal death, long term risk of developing HTN and related disorders |
|
|
HELLP Syndrome
- Definition |
Variant of pre-eclampsia with laboratory evidence of:
- Hemolysis - Elevated Liver enzymes = hepatic dysfunction - Low Platelet counts = thrombocytopenia (<100,000 cells/µL) |
|
|
What is the difference between eclampsia and pre-eclampsia?
|
Eclampsia is the development of seizures in a woman with pre-eclampsia
|
|
|
What can cause hypothyroidism in pregnancy?
|
- Not meeting higher iodine requirement
- Hashimoto's Thyroiditis (presence of Tg or TPO antibodies) |
|
|
What is hypothyroidism in pregnancy associated with?
|
- Deficiency causes goiter
- Severe deficiency causes neurocognitive impairment and mental retardation in offspring |
|
|
What is Hashimoto's thyroiditis in pregnancy associated with?
|
- Recurrent pregnancy loss and preterm delivery
- Presence of Tg or TPO antibodies, causing hypothyroidism |
|
|
What symptoms are associated with gestational hyperthyroidism? Cause?
|
- Transient
- Increased nausea and vomiting (hyperemesis gravidarum) - Arises from TSH receptor stimulation by hCG |
|
|
What are the risks associated with Grave's disease in pregnancy?
|
Increased risk of:
- Abortion - Preterm labor - Low birth weight - Stillbirth - Pre-eclampsia |
|
|
What is the cause of Grave's disease? How do you treat in pregnancy?
|
- Cause: circulating antibodies that cross placenta
- Treatment: several months before pregnancy |
|
|
What is a thyroid storm? Symptoms? Treatment?
|
- Thyrotoxic crisis causes acute exacerbation of hyperthyroidism
- Symptoms: fever, tachycardia, seizures, vomiting - Medical emergency that results in death - Treatment: block thyroid hormones |
|
|
SK is a 28 year old G2P1 pregnant with twins at 16w gestation. She is complaining of excessive nausea and vomiting which she did not experience in her first pregnancy. Which of the following disorders is she likely to be suffering from?
a) Gestational diabetes b) Gestational hyperthyroidism c) Hyperemesis gravidarum d) B and C |
* Both Gestational Hyperthyroidism and Hyperemesis Gravidarum
- Gestational hyperthyroidism is associated with increased nausea and vomiting, called hyperemesis gravidarum, caused by increased TSH receptor stimulation by hCG |
|
|
Which of the following can cause hyperthyroidism in pregnancy?
a) Gestational trophoblastic disease b) Abnormally high hCG c) Multiple gestation d) Graves’s disease e) All of the above |
All of the above
|
|
|
The median age of the population in India is much lower than the median age of the population in Canada. Which is the better parameter to compare maternal mortality in these two countries?
Maternal Mortality Ratio or Maternal Mortality Rate |

Maternal Mortality Ratio
|

