![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
45 Cards in this Set
- Front
- Back
|
What does the overall Wheland intermediate look like?
|
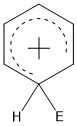
|
|
|
Draw the 3 resonance forms of Wheland intermediate.
|
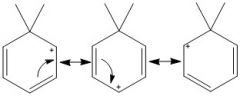
|
|
|
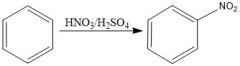
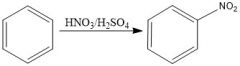
Draw the overall scheme of SEAr.
|
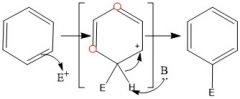
|
|
|
|
|
|
|
|
|
What is the rate determining step of nitration?
|
Addition of the nitro group
|
|
|
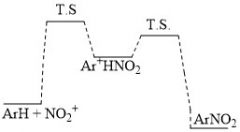
What does the energy diagram of nitration look like?
|
|
|
|
What does the intermediate of nitration look like?
|
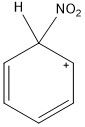
|
|
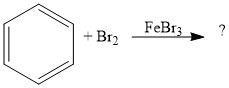
|
|
|
|
|
|
|
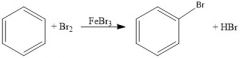
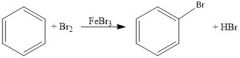
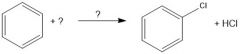
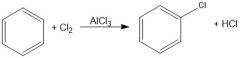
When is FeBr3 necessary for bromination and why?
|
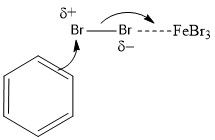
When the aromatic ring is not activated
It is a Lewis base that will draw some of the electron density to one of the bromines in Br2, making the other δ+, a good electrophile |
|
|
|
|
|
|
|
|
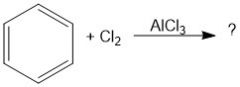
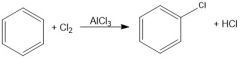
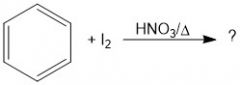
What purpose does AlCl3 serve in chlorination of aromatic compounds?
|
Lewis acid, can break bond of Cl2 for non-activating aromatic rings.
|
|
|
|
|
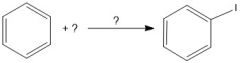
|
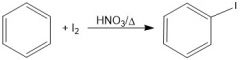
|
|
|
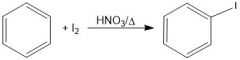
What is the purpose of HNO3/Δ in iodination(?).
|
They oxidize the I2 into 2I+, an active electrophile.
|
|
|
How can a halogenated aromatic compound be used in making a C-C bond?
|
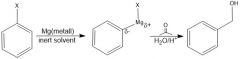
Griiiignard. Grigs are strong nucleophiles - add to electrophiles (aldehyde or ketone)
|
|
|
|
|
|
|
|
|
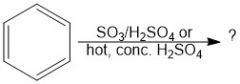
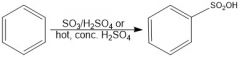
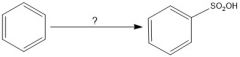
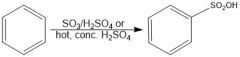
In sulfonation of benzene, which conditions give a faster reaction?
|
SO3/H2SO4 is faster than hot conc. H2SO4
|
|
|
In sulfonation of benzene, which conditions give a slower reaction?
|
Hot conc. H2SO4 is slower than SO3/H2SO4
|
|
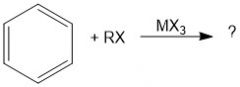
|
|
|
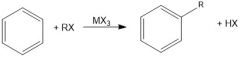
Why is MX3 a necessary reagent in Friedel Crafts alkylation?
|
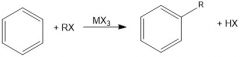
It is a Lewis acid that will break the R-X bond
|
|
|
Which Lewis acid might cause carbocation rearrangements?
|
AlCl3
|
|
|
What Lewis acid might you use to prevent carbocation rearrangement for Friedel Crafts alkylation?
|
FeCl3 is one of many
|
|
|
Draw the general mechanism of Friedel Crafts alkylation
|
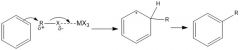
|
|
|
Which R groups are the best for Fried craft alkylation?
|
2° or 3° are best because they are more stable carbocations than 1°
|
|
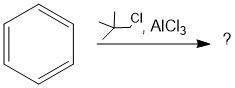
|
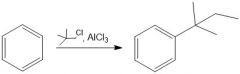
|
|
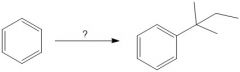
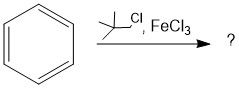
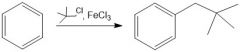
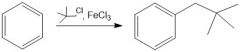
(FC alkylation)
|
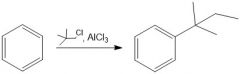
|
|
|
|
|
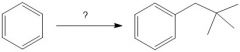
(FC alkylation)
|
|
|
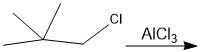
Show how the carbocation rearrangement occurs.
|

|
|
|
|
|
|
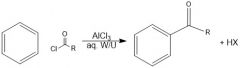
|
|
|
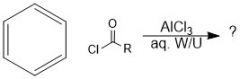
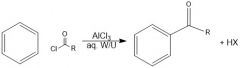
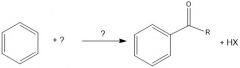
What does an acylium ion look like?
|
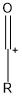
|
|
|
How is an acylium ion made during fried craft acylation?
|
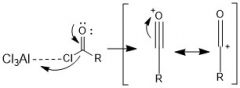
|
|
|
What are the two advantages of FC acylation over alkylation?
|
There are no carbocation rearrangements, and there is no issue with polyalkylation.
|
|
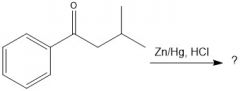
|
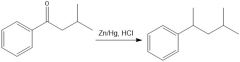
|
|
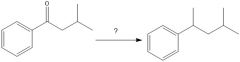
|
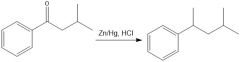
NOT THE ONLY ANSWER DUDE. WHATEVER RELAX
|
|
|
What are some substituents that are deactivating?
|
Halogens, and electron withdrawing groups
|
|
|
Which substituents are deactivating and o/p directing?
|
Halogens
|
|
|
What are some substituents that are strongly o/p directing?
|
-OH, -OR, -NH2, -NR2
|
|

Where do these guys direct other substituents in SEAr?
|
o/p
|
|
|
Why are halogens o/p directing? Shouldn't they be greedy electron sucking ***?
|
They are indeed electron withdrawing through induction, but they also donate their electrons through their lone pairs.
|

