![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
35 Cards in this Set
- Front
- Back
|
Inert |
Lacking the ability or strength to move. |
|
|
Why is Helium Inert? |
Noble Gas |
|
|
Diatomic elements? |
Have No Fear Of Ice Cold Beer
|
|
|
Mendeleev's periodic law? |
"when the elements are arranged in order of increasing atomic mass, certain sets of properties recur periodically"
|
|
|
The periodic law states that.... |
similar properties recur periodically when elements are arranged according to increasing atomic number
|
|
|
Who were the two chemists that came up with the periodic law?
|
Dmitri Mendeleev and Lothar Meyer
|
|

Bohr Model |
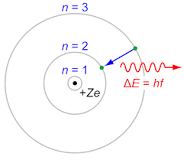
depicts the atom as a small, positively charged nucleus surrounded by electrons that travel in circular orbits around the nucleus
|
|

Quantum Mechanical Model |
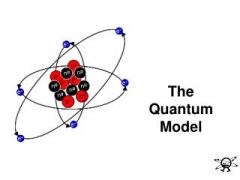
Uses complex shapes of orbitals (sometimes called electron clouds), volumes of space in which there is likely to be an electron.
|
|
|
Quantum Mechanical Model and Bohr Model explain what? |
Theses models explain how electrons exist in atoms and how those electrons affect the chemical and physical properties of elements. |
|
|
Quantum Mechanical Model has such expanatory power that it is rarely questioned today. What are it's applications? |
Its applications include lasers, computers, and semiconductor devices, and it has led us to discover new ways to design drugs that cure disease. THE FOUNDATION OF MODERN CHEMISTRY |
|
|
Interaction of light with atoms help what? |
Help shape both the Bohr and Quantum Mechanical Model. |
|
|
What is light? |
Light is a form of Electromagnetic radiation |
|
|
What light is not? |

Matter - it has no mass |
|
|
What is Electromagnetic Radiation? |
Type of energy that travels through space at a constant speed of 3.0X10^8 m/s |
|

Waterwaves |
Carry energy as it moves through water |
|
|
Waves are characterized by? |

WAVELENGTH |
|
|
What is wavelength? |
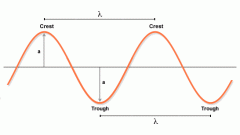
The distance between adjacent wave crests.
|
|
|
What does a wavelength determine? |
COLOR |
|
|
What is white light and what dos it do? |
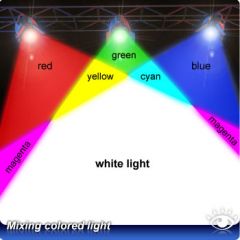
White light, as produced by the sun or light bulb, contains a spectrum of wavelengths and therefore a spectrum of colors |
|
|
Light Waves are also characterized by? |

FREQUENCY |
|
|
Define Frequency |
The number of cycles or crests that pass through a stationary point in one second. |
|
|
Wavelength and frequency are |
Inversely Related - the Shorter the wavelength the higher the frequency. The longer the wavelength the lower the frequency. |
|
|
Results of certain experiments could be explained only by describing light, not as waves, but as_________ |

PARTICLES |
|
|
A particle of light is called |
Photon; a single packet of light energy |
|
|
The amount of energy carried int he packed depends on the wavelength of the light. The shorter the wavelength the __________ the energy. |
Greater |
|
|
The wavelength of electromagnetic radiation determines the ________________ |
the amount of energy carried by one of its photons. |
|
|
ELECTROMAGNETIC SPECTRUM |
The entire range of electromagnetic radiation. |
|
|
Gamma Rays |
Shortest wavelength (and most energetic) photons |
|
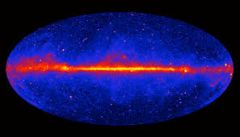
Gamma rays are produced by |
SUN, STARS, and by certain unstable atomic nuclei on Earth. DANGEROUS and can DAMAGE BIOLOGICAL MOLECULES |
|
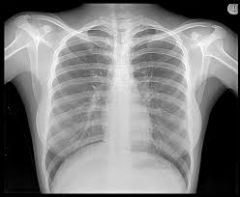
X-rays |
Pass through many substances that block visible light and are therefore used to image internal bones and organs. They can also cause damage to biological molecules. Increase cancer risk. |
|

Ultraviolet or UV light |
most familiar to us as the component of sunlight that produces a sunburn of suntan. Can cause damage to biological molecules. Risk of skin cancer |
|

Visible Light |
ranging from violet to red. Do not damage biological molecules. |
|
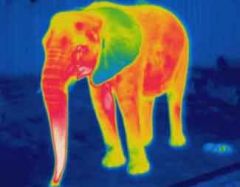
Infrared light |
The heat we feel when we place a hand near a hot object. Warm objects, including human bodies. It is invisible to our eyes, but can be seen through night vision technology |
|
Microwaves |
used for radar and in microwave ovens. Efficient with water and therefore heats substances that contain water. |
|
|
Radio Waves |
Used to transmit the signals by AM and FM radio, cellular telephones, television, and other forms of communication. |

