![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
63 Cards in this Set
- Front
- Back
|
What is the epidemiology of epilepsy?
|
~3 million individuals in US have epilepsy (~1% of the total population)
- 20% are over age 65 (twice the young adult rate) - 9% in nursing home residents > 180,000 new cases annually - Bimodal distribution - U-shaped curve (youngest and older are more highly affected) |
|
|
What is a seizure?
|
the clinical manifestation of an abnormal and excessive excitation of a population of cortical neurons
|
|
|
What is epilepsy?
|
a tendency toward recurrent seizures unprovoked by systemic or neurologic insults
*by contrast, an individual with precipitous drop in blood pressure would also have a seizure but this is provoked and therefore not epilepsy |
|
|
What is epileptogenesis?
|
sequence of events that converts a neuronal network into a hyperexcitable network that then spontaneously generates seizures
|
|
|
What are the phases of a seizure?
|
1) Aura - The earliest experienced symptoms that predict an imminent larger seizure to follow. This actually represents the early ictal phase, affecting a sufficiently small cortical region so that consciousness is unaffected and memory of the aura is generally intact as well.
-- Temporal lobe seizure auras involve rising epigastric distress, deja vu character or olfactory/gustatory hallucinations 2) Ictus/Ictal - The period of the actual seizure 3) Post-ictal - The period of abnormal behavior that follows the ictus (e.g. confusion, paresis, lethargy, occasionally focal findings such as weakness) 4) Inter-ictal - The baseline phase in between seizures (most diagnostic tests are performed during this phase) |
|
|
What are the general types of seizures?
|
Focal/Partial
- Arises from a particular region of the cortex at onset -Can be complex or simple Generalized - Arises diffusely (bilaterally synchronous) - Two subtypes -- Primary (starts generalized) or Secondary (starts as focal and then spreads into both hemispheres/becomes generalized) -- These two can only be differentiated by knowledge of onset pattern/through prediction with clinical/EEG data |
|
|
What is the phenotype of a focal seizure?
|
Signs and symptoms reflect local cortical functions
- Motor, visual, sensory, limbic - Seizure propagation can lead to phenotypic evolution ( = semiology of the seizure) Classification based upon signs & symptoms - Motor, sensory, autonomic, psychic - Simple vs. complex partial (LOC) -- Depends on whether consciousness is altered (as seen in complex partial seizures where patient has amnesia of the process) Classification based upon locale of onset - Frontal (even supplementary motor), or parietal, or temporal (even mesial temporal = area closest to the hippocampus), etc. |
|
|
What is the phenotype of a generalized seizure?
|
Non-convulsive:
- Absence (“petit mal”) = primary generalized “staring spells” with bilateral involvement -- Patients sometimes falsely attribute “petit mal” to complex partial seizures - generalized are shorter, with fewer post-ictal phenomena, but differentiation is typically done with EEG Convulsive: - Generalized Tonic-Clonic (“grand mal”) - repetitive jerking movements - Myoclonic - brief jerking movements (synchronous) - Tonic - sudden assumption of a dystonic posture - Atonic - loss of muscle tone (patient may fall to the ground) |
|
|
What is included in an epilepsy syndrome?
|
Seizure type(s)
Age of onset Associated deficits Associated EEG patterns Etiology |
|
|
What is the most commonly used classification system for epilepsy?
|
ILAE '89 - very simple (and deficient, as detailed below)
- Focal -- Symptomatic (secondary) eg. Post-traumatic temporal lobe epilepsy -- Idiopathic (primary) eg. Autosomal dominant temporal lobe epilepsy with auditory features - Generalized -- Symptomatic (secondary) eg. tuberous sclerosis -- Idiopathic (primary) e.g JME Deficiencies: - Idiopathic epilepsies tend to be genetic, so as the genetic roots are found, they are not idiopathic any more - Symptomatic epilepsies may be secondary to some brain insult, but many times this insult is unknown Revisions include using anatomical precision first (localization), followed by age and associated features, and etiology |
|
|
What are causes of seizures?
|
Metabolic derangements
- hyponatremia, hypo- or hyperglycemia, hypocalcemia, hypomagnesemia Toxins eg. stimulants Withdrawal e.g from alcohol or benzodiazepines Fever CNS trauma, infection, ischemia, hemorrhage |
|
|
What are causes of epilepsy?
|
Focal CNS injuries
- trauma, tumor, hemorrhage, infection, ischemia, inflammation Neurodegenerative illnesses CNS developmental abnormalities - Focal cortical dysplasias, lissencephalies, PVNH, hemi-megalencephaly, tuberous sclerosis, etc. Genetic - ADFLE, GEFS+, BFNC, JME, JAE, etc - Fall into a variety of categories that control early neurogenesis and neuronal migration |
|
|
Is epilepsy etiology age-related?
|
Generally yes.
First two decades: - Genetic - Early Life CNS Insults (infection, vascular or CNS dysgenesis) In the elderly: - Vascular (e.g. stroke, 30-50%) - Neoplasia (10-15%) - Neurodegenerative - Toxic-metabolic - Drug/ETOH withdrawal |
|
|
Are seizures the result of excessive neuronal excitability or diminished neuron inhibition or both?
|
Depends on the type of seizure
|
|
|
Is epilepsy an emergent property of neuronal circuits or individual neurons?
|
Circuits
|
|
|
What is the time course of epileptogenesis from CNS injury?
|
Acute symptomatic seizures after CNS insult -> possible later epilepsy (variable)
No immediate seizures -> "silent period" of weeks/months/years after injury -> delayed seizures (unprovoked) |
|
|
What can lead to the hyperexcitable networks in epilepsy?
|
- Excitatory axonal "sprouting" that leads to reverberant circuits that are hyperexcitable
- Inhibitory neuron loss - Inhibitory neuron inactivation from loss of the excitatory neurons that drive them |
|
|
What is the kindling model of epileptogenesis?
|
Repeated subconvulsive stimuli (in mice)
--> Electrical afterdischarges --> (Eventually) stimulation-induced clinical seizures --> (Even more eventually) spontaneous seizures/epilepsy Uncertain applicability in humans |
|
|
What are the pathological findings in epilepsies?
|
- Hippocampal sclerosis (often w/ radiologic evidence of mesial temporal sclerosis [cell loss vs. synaptic reorganization])
- Cortical dysplasia and/or heterotopias - Polymicrogyria, pachygyria, other gyral abnormalities - Possibly nothing! |
|
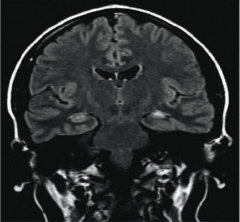
What are the features above and what do they indicate?
|
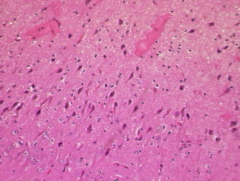
Hippocampal atrophy on the left
FLAIR hyperintensity Both indicate hippocampal sclerosis Close-up of CA1 region is shown above and shows neuronal loss |
|
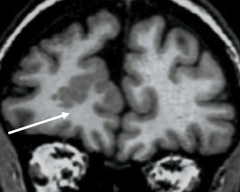
What is indicated in the image?
|
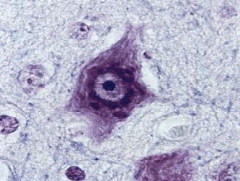
Cortical dysplasia - gray matter in an abnormal area
Balloon cell shown above can be seen in the region of dysplasia |
|
|
What are the tools in diagnosing epilepsy?
|
- History
- Exam - LTM (Longterm video/EEG monitoring - can be at home or in the hospital) - Imaging: -- CT (ubiquitous) -- MRI (more sensitive) -- Additional studies (SPECT, PET, MEG, MRS) for pre-surgicals - EEG |
|
|
What is an EEG?
|
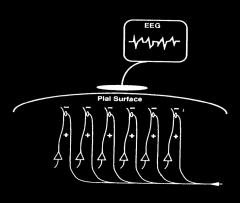
Graphical depiction of cortical electrical activity
- Electrical field generated by similarly oriented pyramidal cells in cortex (layer 5) and detected by scalp electrode Records voltage differences from points on the scalp Relates to synchronous activation of apical dendrites of pyramidal neurons in upper neocortical laminae by excitatory afferent inputs |
|
|
What are abnormalities in an EEG?
|
Spikes, sharp waves, spike-wave complexes, polyspikes
- Transient epileptiform abnormalities associated with risk of seizure - Focal, lateralized, or generalized Background abnormalities - Asymmetry - Slowing → indicates encephalopathy Focal slowing / diffuse slowing |
|
|
What is the yield of an EEG in diagnosing epilepsy?
|
Yield is 50% with single routine study, increases with repetition
|
|
|
What are the steps taken if an EEG does not confirm diagnosis?
|
Repeat EEGs
Add sphenoidal leads and/or sleep deprivation to improve yield Ambulatory 24-hour (or longer) studies (LTM) Inpatient long term video-EEG monitoring (LTM) |
|
|
What are the types of EEG for refractory patients seeking surgical treatment?
|
Phase I: Scalp video-EEG monitoring
If moving on to surgical treatment: Phase II: Invasive monitoring (grid/depth electrodes) |
|
|
What are the differential diagnoses for epilepsy?
|
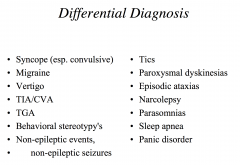
|
|
|
What is the difference between a spell and a seizure?
|
Seizure implies disrupted cerebral physiology with hyperexcitable, hypersynchronous neuronal activity
Spell is less specific of pathophysiologic mechanism Patients often use ‘seizure’ with less precision to describe many types of transient attacks ‘Spell’ (or ‘event’) is preferable if etiology is unknown |
|
|
What is the difference between a seizure and syncope?
|
A. Setting
- Sz: Random, often in sleep, may be seated, not situational - Sy: Often situational (pain, emotion, dry), upright, voiding B. Prodrome - Sz: None, or typical aura (GI, déjà vu, sensorimotor) - Sy: Nausea, pallor, clammy, visual graying, altered pulse C. Ictus - Sz: Rigid fall, injuries, incontinence (most helpful clinical feature), clonus - occ. focal - Sy: Flaccid fall, rare injury, brief + generalized jerking D. Confusion - Sz: Prominent, minutes to hours, occasional Todd’s paresis (temporary paralysis following seizure) - Sy: Mild, fleeting, no paresis, “shaken up” |
|
|
What is status epilepticus? What is acute treatment?
|
Ongoing seizure activity >10-15 minutes (typical seizure is 1-2 minutes, 5 minutes is very long already)
Recurrent seizures without interval recovery to neurologic baseline Convulsive (generalized [tonic-clonic], focal [epilepsia partialis continua]) or non-convulsive (generalized [absence], focal [complex partial]) Can result in neural injury (excitotoxicity, metabolic stress) due to the length of time (time=brain) Acute treatment: Benzodiazepine or Phenytoin IV |
|
|
What are the standard drug treatments for epilepsy?
|
Benzodiazepines (IV or per rectum)
First-line IV AEDs (phenytoin, fosphenytoin) Second-line IV AEDs (barbiturates, others) Sedative agents (pentobarbital, midazolam, propofol) |
|
|
What are the response rates for epilepsy patients to AEDs?
|
30% of patients are refractory to AEDs, and among the population that are not refractory:
- Response may improve for patients who sustain recurrences after a first AED by giving them a second AED, or duotherapy |
|
|
What are non-AED treatments for epilepsy?
|
Lifestyle modifications - more sleep, stress reduction, avoidance of substance abuse/photosensitive stimuli
ACTH for infantile spasms IV IgG - case reports Hormonal approaches Surgery (lobectomy [temporal most common], lesionectomy) Palliative surgeries, radical surgeries (hemispherectomies, callosotomy [cutting the corpus callosum]) Vagal nerve stimulation Ketogenic diets (pediatric) - lipid rich diet that can benefit seizures Experimental methods (AEDs, CNS stimulation) |
|
|
What are the actions of antiepileptic drugs (AEDs)?
|
Important:
Na+ channel blockade - decreases action potential frequency Increase GABAnergic transmission (+ GABA_A receptor activity, + GABA synthesis/release, - GABA reuptake/degradation) - increases inhibition Other: Ca2+ channel blockade - decreases (1) thalamocortical reverberations (T-type), (2) cortical excitation (L-type), (3) neurotransmitter release (N-type) Decrease glutaminergic transmission (block receptors, decrease synthesis/release) - decreases excitation |
|
|
What is the method of action of sodium channel blockers?
|
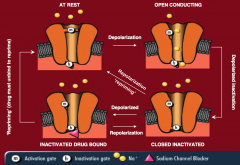
Normal sodium channels have two gates, an M gate and an H gate, and in the normal inactive form, the M gate is closed and the H gate is open
Depolarization opens the M gate, and the sodium flows through, and eventually the H gate closes. The channel eventually repolarizes and returns to the normal inactive state for the next depolarization With a sodium blocker, the H gate is kept closed, and repriming and returning to normal is prevented, with the main effect being the prevention of rapid hyperexcitability of the channel |
|
|
What are the effects of sodium blockers on seizures?
|
There is no marked impact on normal firing of action potentials.
Inhibits repetitive firing behavior of neurons Less effect on threshold for seizure, but more of an effect on suppressing spread of epileptic activity |
|
|
What are examples of sodium channel blockers?
|
Phenytoin
Oxcarbazepine Lamotrigine Topiramate (in part) Carbamazepine Lacosamide Valproate (in part) |
|
|
What are examples of GABA_A receptor drugs?
|
Phenobarbital - GABA agonist
Primidone - metabolite is phenobarbital Benzodiazepines (diazepam, lorazepam, clonazepam, clobazam) - GABA agonists Tiagabine - GABA reuptake inhibitor Vigabatrin - GABA catabolism inhibitor |
|
|
What is the structure of the GABA_A receptor?
|
Pentameric structure - 2 alpha, 2 beta, 1 gamma subunits
Cl- permeable channel GABA binding domain is on the beta subunit, and also contains binding domains for benzodiazepines (agonist), barbiturates (agonist), and neuroactive steroids (agonists and antagonists) |
|
|
What is the mechanism of action of AEDs on GABA receptors?
|
GABA receptors normally open to allow chlorine to flow through and dampen excitation
Benzodiazepines and barbiturates modulate this action, and can either increase the frequency of channel opening or extend the time that the channel remains open. Binds in different site than GABA. |
|
|
What are the types of Ca2+ channels?
|
L, N, T, P/Q, R
T-type are low-voltage activated channels - Key for bursting behavior of thalamo-cortical oscillators: these are relay cells that relate for how these all areas of the brain relate to each other. This is thought to be one of the important targets for therapy because they think that the reason the cortex gets stuck in these 3 hz spike and wave pattern relates to this circuit (thalamic neuron, cortical neuron and interneuron). - Relevant to absence seizures - valproate, ethosuximide are effective and interact with these channels L and N are high-voltage activated (HVA) channels - Presynaptic channels involved in transmitter release → blocking these can decrease how much glutamate is released into the synapse. |
|
|
What are the glutamate-based treatment mechanisms?
|
Blockage of glutamate transmission:
APMA/Kainate receptor blockage - Topiramate NDMA receptor blockage - Felbamate, Ketamine, Dextromethorphan, Mg2+, Zn2+ Blockage of calcium channels possibly by blocking L and N type Ca2+ channels (high-voltage activated), blocking release of glutamate |
|
|
What are the broad categories of AEDs based on function?
|
Broad Spectrum - can treat both generalized and partial seizures (valproate, lamotrigine, topiramate, zonisamide, levetiracetam[, felbamate, rufinamide])
Infantile spasms - ACTH, vigabatrin Absence seizures - ethosuximide Partial/focal seizures - phenytoin, carbamazepine, phenobarbital, gabapentin, tiagabine, oxcarbazepine, pregabalin, lacosamide Note: Newer drugs are more broad spectrum |
|
|
What are the general pharmacologic parameters?
|
Absorption
Route(s) of administration Distribution Half-life (T1/2), dosing frequency Protein Binding Hepatic enzyme induction, inhibition Clearance mechanisms, metabolites Mechanism of action Tolerance, dependence Time to reach therapeutic range |
|
|
What are the pros and cons of the older AEDs?
|
Good:
Familiar Levels available Inexpensive IV and PO Long-term risks known Bad: More side effects More interactions: drug-drug interactions because they affect the hepatic enzyme pathways that are critical to the processing of other drugs or hormones and bind to protein. More blood monitoring : can affect liver and blood counts Narrow therapeutic zone Long-term risks known |
|
|
What is phenytoin? What are its adverse effects?
|
Most widely used anticonvulsant
Blocks sodium channels, and used for partial onset seizures and status epilepticus PO or IV, QD or BID w/ long half-life Adverse effects: - It is highly protein bound and displaces other bound drugs - Induces Cytochrome P450 - Zero-order kinetics at higher levels (meaning that the drug will go from having a linear effect of dosing to having an exponential increase in blood concentration per dose rate increase) - Other effects: -- Allergic reactions, rarely severe (Stevens-Johnson) -- Gum hypertrophy -- Cerebellar toxicity -- Bone health -- Peripheral neuropathy -- Bone marrow effects (rare) |
|
|
What are barbiturates? What are their adverse effects?
|
GABA agonists that are used for partial onset seizures
PO, IV Adverse effects: Hepatic (Cytochrome P450) Enzyme Inducer Sedation, mood effects Dependence Bone health Allergic risk |
|
|
What are new formulations to the older AEDs that can improve treatments?
|
Longer-acting drugs - better compliance with better blood levels (e.g. carbamazepine, valproate , amotrigine, levetiracetam)
Alternative routes of administration - rectal diazepam can be used to prevent hospitalization in patients with flurries of seizures - inhaled midazolam (experimental) |
|
|
What are the pros and cons of the newer generation AEDs?
|
Benefits
- Fewer drug interactions: less hepatic impact, less protein binding - Fewer side effects (possibly) - Broad spectrum (many) - Most are BID or less - Less need for blood monitoring - New and multiple mechanisms of action - More are renally cleared Potential Problems - Unclear long-term risks (theoretically fewer) - Costly (until generic available--variable) |
|
|
What are the effects of AEDs on contraception?
|
AEDs that result in enzyme contraceptive failure/diminishing: phenytoin, carbamazepine, barbiturates, oxcarbazepine (dose dependent), topiramate (dose dependent), rufinamide
AEDs that have no effect on contraceptives: gabapentin, valproate, lamotrigine (probably insignificant), levetiracetam, tiagabine, zonisamide, pregabalin, lacosamide |
|
|
What are the effects of AEDs on pregnancy?
|
Lamotrigine is better than the rest of the AEDs, but still doubles the risk compared to women not on AEDs
Carbamazepine and Valproate can cause neural tube defects (spina bifida), and Valproate in particular is particularly bad with fetal malformations Summary: Lamotrigine - good, carbamazepine - bad, valproate - really bad. (Lamotrigine good for OCP use and pregnancy) |
|
|
What are the times used to reach an effective dose for AEDs?
|
Fast (<1 week):
Phenytoin Valproate Barbiturates Levetiracetam Zonisamide Intermediate (2 weeks): Carbamazepine Oxcarbazepine Topiramate Ethosuximide Gabapentin Pregabalin Lacosamide Slow (6+ weeks): Lamotrigine (slow dosage reduces the chance of Stevens Johnson Syndrome) Tiagabine Rufinamide |
|
|
What are the pros and cons of generic AEDs?
|
Context: There may be a dozen different generic formulations for any particular AED. Pharmacies can switch monthly to save on cost → they find a cheaper generic supplier. For the FDA all the Generics have to do is achieve 80-125% of bioavailability of brand name. However if you flip from generic to generic you could theoretically have a range of 80%-125%. This may permit significant theoretical swings in blood levels on a month to month basis.
Pro: Cost Con: Consistency of blood level; a single seizure can create much havoc and morbidity, even mortality |
|
|
What is the goal of epilepsy therapy?
|
“no seizures, no side effects”
- Achieve complete control of seizures - Minimize side effects Permit normalization of patients’ lives: vocation and education Patients will generally require long-term medication |
|
|
What are the basic guidelines for AED use?
|
- Therapy is tailored to the seizure type
- Work with one drug at a low-dose, and titrate up depending on the suggested speed (slow, fast) - Simple regimens are good - Education of patients on compliance and AEDs for prevention - Balance efficacy, side effects, quality of life, and depends partly on what the patient wants |
|
|
What are the factors for selecting an AED?
|
Seizure Type
Efficacy (little head-to-head data) Safety Side Effect Profile (Acute + chronic) Impact on hormones, pregnancy Dosing Frequency Speed of Achieving Therapeutic Range Interactions Cost |
|
|
What is Juvenile Myoclonic Epilepsy? What are its characteristics and treatments?
|
Characterized by mixed generalized seizure types including myoclonic, GTC, and absence, usually beginning in childhood or early adolescence.
The EEG shows bilaterally synchronous generalized pattern with spike and polyspike and slow wave activity at >3 Hz, often 4-6 Hz (fast spike wave pattern). Usually respond well to broad-spectrum AEDs. Often a lifelong trait can be well managed. |
|
|
What are considerations with treating seizures in elderly patients?
|
Need to be careful with dosing and with rapid titration
Lower doses, slower titration Patients with many other drugs would probably do better with drugs like gabapentin, lamotrigine, levetiracetam due to minimal drug interactions However, few drugs have been tested in elderly .populations |
|
|
Why do we treat epilepsy with monotherapies?
|
Monotherapy minimizes interactions, complexity and 50% of patients will be controlled with 1st AED. Successfully we usually aim for low-medium therapeutic level or dose and then advance steadily until achieving seizure-freedom or unacceptable side effects.
|
|
|
Why can monotherapies not work?
|
Incorrect diagnosis
Drug-resistant condition Underlying progressive neurologic disorder Inappropriate AED Suboptimal dosing Intolerable side effects Noncompliance Drug interactions Non-prescriptive interactions |
|
|
When do you stop an AED?
|
At least two years seizure free
EEG may be useful to gauge risk May be riskier if the substrate for seizure persists (abnormal scan or exam): you need to have a discussion with the patient to be comfortable takign a risk of having another seizure. A discussion of risks and benefits with patient (plus family at times) and the neurologist Easier to taper from 3 AEDs to 2 than from 2 to 1, and certainly easier than tapering entirely off AEDs **Go slowly, over weeks to months. |
|
|
Why do we treat pregnant mothers with epilepsy despite the hazards from AEDs?
|
The seizures themselves also pose hazards to pregnancy
|

