![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
74 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Which is the best chemical excipient for treating lead poisoning?
|
edetate calcium disodium
|
|
|
|
Water
|
Why is it a good solvent?
1. High dielectric constant 2. Tendency of binding with ions to form "hydrated ions" Water impurities: Carbon dioxide, calcium, magnesium, iron, oxygen, nitrogen, inorganic salts |
|
|
|
Purified Water, USP
|
More free of solid impurities than drinking water (less than 1mg of total solids/ 100 mL) with no added substances (preservatives)
Used in oral and topical aqueous dosage forms. Not used in parenteral dosage forms. |
|
|
|
Three ways to prepare purified water, USP
|
1. Distillation - first and last 10 % distillate must be discarded (most expensive)
2. Ion -exchange - water passes through cation and anion exchange resins which are made out of sulfonated cross-linked polystyrene. Water purified this way is called deionized or demineralized water. 3. Reverse osmosis - When a concentrated solution and a dilute solution (or water) are placed in between a semi-permeable membrane, water from a dilute permeates through the membrane (referred to as osmosis) and then osmotic pressure builds up in a concentrated solution. However, when pressure above the osmotic pressure exerts on the concentrated solution, water flows reversely (referred to as reverse osmosis). This process removes 90-99% of all ions, all bacteria, pyrogens, organic molecules. |
|
|
|
Alcohol (ethanol)
|
Alcohol USP: 94.9 to 96.0% C2H5OH by volume at 15.56 degree celsius
Used as a primary solvent to dissolve water-insoluble compounds. |
|
|
|
Percent alcohol recommended according to age
|
Under 6 years old - 0.5% alcohol
Age 6-12 years old - 5% alcohol Age 12 years and older - 10% alcohol |
|
|
|
Glycerin USP
|
Viscous and used as a stabilizer, a humectant (a substance that promotes retention of moisture), and an auxiliary solvent with water or alcohol.
|
|
|
|
Propylene Glycol USP
|
viscous and frequently used for glycerin substitute. Humectant (prevent evaporation), preservative.
|
|
|
|
Isopropyl rubbing alcohol
|
about 70% isopropyl alcohol by volume. Used for a germicide for needles and syringes for diabetic patients. FOR EXTERNAL USE ONLY.
|
|
|
|
Polyethylene Glycol (PEG) NF
|
PEG 400 is the most common liquid PEG used in pharmaceutical dosage forms. PEG <600 = humectant
|
|
|
|
Oils
|
Almond Oil NF, Castor Oil USP, Corn Oil NF, Cottonseed Oil NF, Heavy Mineral Oil USP, Light Mineral Oil NF, Olive Oil NF, Peanut Oil NF, Safflower Oil USP, Sesame Oil NF, and Soybean Oil USP.
|
|
|
|
Syrups
|
Concentrated aqueous preparations of a sugar or sugar-substitute with or without flavoring agents and medicinal substances. They can serve as pleasant-tasting vehicles for active drugs.
(1) the sugar, usually sucrose (60 to 80%), or sugar-substitutes (e.g., dextrose, sorbitol [no less than 64%], glycerin and propylene glycol) for sweetness and viscosity.The high concentration of sucrose is resistant to microbial growth due to the scarcity of water |
|
|
|
Noncaloric sweeteners
|
Aspatame is 200 times sweeter than sugar
Sodium saccharin is 250-500 times sweeter than sugar |
|
|
|
Cyclamate
|
Sugar substitute
It is metabolized in the digestive tract and its byproducts are excreted by the kidneys. |
|
|
|
Aspartame
|
Sugar substitute
Breaks down into phenylalanine, aspartic acid and methanol. Heat- sensitive (NutraSweet). Equal is a brand containing aspartame, dextrose and maltodextrin. |
|
|
|
Sucralose
|
Sugar substitute
600 times as sweet as sucrose (table sugar) Twice as sweet as saccharin 3.3 times as sweet as aspartame. It is stable under heat and over a broad range of pH conditions. Therefore, it can be used in baking or in products that require a longer shelf life. |
|
|
|
Acesulfame potassium
|
Sugar substitute
Structurally similar to saccharin, 130X much sweeter than sucrose, excreted by the urine unchanged. |
|
|
|
Stevia
|
natural plant extract-nontoxic, safe, and 30X sweeter than cane sugar or sucrose.
|
|
|
|
Preservatives For Pharmaceutical Products
|
Ethyl alcohol
Propylene glycol Glycerin Benzoic acid /sodium benzoate/potassium benzoate Sorbic acid /potassium sorbate Methylparaben/Propylparaben Benzyl Alcohol (NO NEONATAL PRODUCTS) Phenylmercuric Acetate /Phenylmercuric Nitrate /Thimerosal |
|
|
|
Quaternary ammonium salts
|
Preservatives used in oral products but are used primarily in topical and ophthalmic products.
Benzalkonium Chloride Cetylpyridinium Chloride Benzethonium Chloride Chlorobutanol (heat sensitive) |
|
|
|
Zero order reactions only
|
The rate of degradation is independent of drug concentration.
The rate of degradation is always constant. One can calculate 100% degradation time. After three half-lives, there will be no drug left. Half life is dependent on initial drug concentration. Zero order degradation follows a linear pattern. |
|
|
|
First order reactions only
|
The semi-log plot of concentration vs time gives a straight line.
The half-life is constant and independent of initial concentration. |
|
|
|
Indicates that degradation is not first order. (The reaction could be zero order but one cannot tell for sure)
|
The semi-log plot of concentration versus time does not give a straight line.
|
|
|
|
Applies to both zero and first order reactions
|
The semi-log plot of k (rate constant) versus 1/T is linear.
The unit of the rate of drug degradation is g L ^-1 sec ^-1 |
|
|
|
Maceration
|
Extraction Methods for Preparing Solutions
A process in which crude drugs in the plant tissues are soaked and dissolved in the menstruum (extracting solvent or solvent mixture) like a tea bag. Benzoin, aloe and totu at 15 - 20C for 3 hrs. |
|
|
|
Percolation
|
Extraction Methods for Preparing Solutions
A process in which a comminuted drug is placed in a column (termed a percolator) and a suitable solvent pass slowly through it: fluidextracts (1g/mL). |
|
|
|
Aromatic Waters
|
Clear, aqueous solutions containing saturated volatile oils or other aromatic or volatile substances. Orange flower oil, peppermint oil, rose oil, camphor, etc. They may be used for perfuming and/or flavoring.
|
|
|
|
Spirits
|
Alcoholic or hydroalcoholic solutions of volatile substances. The concentration of alcohol is usually over 60%. They are used pharmaceutically as flavoring agents and therapeutically if the aromatic substances are medicinal: aromatic ammonia spirit, camphor spirit, compound orange spirit, and peppermint spirit.
|
|
|
|
Liniments
|
Alcoholic or oleaginous solutions or emulsions of drug substances intended for external use to the skin with rubbing. Alcoholic or hydroalcoholic vehicles are useful for rubefacient, counterirritant, or penetrating action. Oleaginous vehicles are primarily for massage
|
|
|
|
Collodions
|
Liquid preparations consisted of pyroxylin (soluble gun cotton) dissolved in a solvent mixture usually composed of ether and alcohol (exceedingly flammable) with or without medicinal substances. They are intended for external use. Upon applying to the skin with a brush, the solvent evaporates, leaving a film which provides an occlusive dressing to the skin: collodion (inflexible), flexible collodion (2% camphor, 3% castor oil), and salicylic acid (keratolytic agent: An agent that dissolves or breaks down the outer layer of skin (keratins)) collodion. They hold the edges of an incised wound together.
|
|
|
|
Tinctures
|
Alcoholic or hydroalcoholic solutions prepared from chemical substances or vegetable materials. The alcohol content ranges from 15 to 80%.
Many topical tinctures are employed for anti-infectives applied to the skin and contain a dye to delineate the application area: Iodine Tincture (2% I2; 2.4% NaI), Compound Benzoin Tincture (10% benzoin; 2% aloe; 8% storax; 4% tolu balsam), and Thimerol Tincture |
|
|
|
molecular solutions of small ions and molecules
|
Particle size : below 10 A or 1 nm
Ca+2 (1 A), SO4-2 (3 A), sucrose (4.5 A) Characteristics : too small or lower limit of resolution of electron microscope; high diffusion rate, pass through filter and dialysis, cytoplasmic and glomerular membranes; high osmotic pressure Examples : NaCl or sucrose solution vitamin, A acetate in cotton seed oil; cholesterol in olive oil |
|
|
|
colloidal dispersions and solutions of macromolecules
|
Particle size: 20 A (2 nm) to 1 microliter
Characteristics: visible in electron microscope and often in ultramicroscope; invisible in light microscope; low diffusion rate; undergo Brownian motion; pass through filter paper but retained by ultrafilter Examples: bentonite magma; suspensions of virus and solutions of albumin, gelatin, acacia, NaCMC, Povidone in water; polystyrene in benzene; surfacatant micelles |
|
|
|
coarse dispersions
|
Particle size : greater than 1 microliter
Characteristics: visible in light microscope; too big to diffuse or undergo Brownian motion; settle or rise; retained by filter paper; negligible osmotic pressure Examples: most pharmaceutical suspensions and emulsions; erythrocyte in blood |
|
|
|
Dispersed system
|
An immiscible or insoluble system in which one substance, referred to as the discontinuous dispersed phase, is distributed as particles throughout another substance, termed the continuous dispersion (dispersing) medium
|
|
|
|
Disperse system classification
|
emulsions (liquid/liquid),
suspensions (solid/liquid) aerosols (solid/gas or liquid/gas) |
|
|
|
Anionic surfactants
|
widely used due to their inexpensive and
limited to external use: sodium palmitate, potassium or sodium oleate, sodium stearate, sodium lauryl sulfate, sodium dodecylbenzenesulfonate, sodium dioctyl sulfasuccinate, triethanolamine stearate, bile salts. |
|
|
|
Cationic surfactants
|
quaternary ammonium compounds and also used as preservatives: benzalkonium chloride, benzethonium chloride, and cetylpyridinium chloride.
|
|
|
|
Ampholytic surfactants
|
consist of both positively and negatively charged groups in a molecule. Anionic at high pH and cationic at low pH.
Ex: dodecyldimethylbetaine, dimethyldodecyl- ammoniumpropane sulfate, and lecithin |
|
|
|
Nonionic surfactants
|
compatible with other compounds than anionic or cationic surfactants and less sensitive to pH or electrolytes. They are low in toxicity and irritancy and used widely for oral and parenteral applications. However, they are expensive.
Esters, alcohol, sorbitan esters, polysorbate, natural nonionic sufactants, nonionic polymers |
|
|
|
Esters
|
nonionic surfactants
glyceryl tristearate and polyethylene 40 glycol stearate |
|
|
|
Alcohol
|
nonionic surfactants
polyoxyethylene laury alcohol |
|
|
|
Sorbitan esters
|
nonionic surfactants
(Span series): sorbitan trioleate (Span 85), sorbitan sesquioleate (Span 83), sorbitan monooleate (Span 80), sorbitan monostearate (Span 60), sorbitan monopalmitate (Span 40), and sorbitan monolaurate (Span 20). |
|
|
|
Polysorbate
|
nonionic surfactants
Tween seires): polyethylene sorbitan monooleate (Tween 20), Tween 40, Tween 60, and Tween 80 |
|
|
|
Natural nonionic surfactants
|
nonionic surfactants
stearyl alcohol, cetyl alcohol, wool fat (Lanolin) or wool wax and its derivatives, wool alcohols, and cholesterol. |
|
|
|
Nonionic polymers
|
nonionic surfactants
Pluronics (Poloxomer) |
|
|
|
Methods of preparing Primary Emulsions
|
1. Dry Gum (Continental) Method (4:2:1 method)
2. Wet Gum (English) Method 3. Bottle (Forbes Bottle) Method 4. Soap Method |
|
|
|
Dry Gum (Continental) Method (4:2:1 method)
|
Oil (4 parts) and acacia gum (1 part) are triturated in a perfect dry mortar (Wedgewood), having rough surface (porcelain), until the gum is distributed uniformly in the oil.
Two parts of water are added at once and the mixture is triturated quickly to form the creamy white primary emulsion for 5 minutes. The remaining water and other ingredients are added to the primary emulsion with stirring to finish the product. High concentration of electrolytes tends to crack an emulsion and should be added last. The gum is insoluble in alcohol. Thus alcohol should not be added to the primary emulsion and would be after all other ingredients had been added. The proportions of primary emulsion can be adjusted: 3:2:1 or 2;2;1 for volatile oils, light mineral oil, and linseed oil. |
|
|
|
Wet Gum (English) Method
|
A primary emulsion consists of 4:2:1 (oil;water:acacia gum). But the order of mixing is different from the dry gum method.
Two parts of water and 1 part of acacia gum are triturated until a smooth, viscous mucilage is obtained. Oil is added slowly in portions with continuous trituration to form a primary emulsion. The remaining water and other ingredients are mixed as the dry gum method. |
|
|
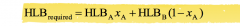
|

|
Hydrophile - Liphophile Balance (HBL)
HLB required = required HLB of the dispersed phase HLBA = HLB value of surfactant A HLBB = HLB value of surfactant B Xa = the weight fraction of surfactant A in the surfactant mixture More hydrophilic surfactants exhibit high HLB values (>10) whereas more lipophilic surfactants have low HLB values (1 to 10) |
|
|
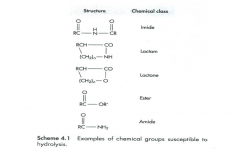
Chemical groups susceptible to hydrolysis
|
|
|
|
|
chelating agents
|
Trace metal ions (i.e., divalent and trivalent) originating from the drug, solvent container, and stopper can catalyze an oxidation reaction and are a constant source of difficulty in preparing a stable solution of oxidizable drugs
Must incorporate: EDTA (ethylene diamine tetra acetic acid) [Edetic acid NF] Edetate disodium Edetate calcium disodium Citric acid Tartaric acid |
|
|
|
Water – soluble anti-oxidants
|
Inorganic:
Na sulfite Na formaldehydesulfoxylate Na thiosulfate Na bisulfite Na metasulfite |
Organic :
Ascorbic acid Thiourea Thioglycerol Thioglycolic acid |
|
|
Oil – soluble anti-oxidants
|
Hydroquinone
Alpha- tocopherol Dilauryl thiodipropionate Propyl gallate Butylated hydroxy toluene Butylated hydroxy anisole Norhydroguaiaretic acid Ascorbyl palmitate |
|
|
|
Stabilization of Drugs Against Oxidation
|
(a) Use hydrogenated fats and oils of fats and oils in formulations.
(b) Replace air with inert gas (e.g., nitrogen). (c) Incorporate anti-oxidants in the formulation. (d) Use a metal – free solvent or incorporate chelating agents in the formulation. (e) Exclude light (i.e., use colored bottles) (f) Keep medication cold. |
|
|
|
Stabilization of Drugs Against Hydrolysis
|
(a) Adjust the pH of the final dosage form to obtain the lowest rate of degradation (or rate constant) [acid – base specific hydrolysis].
(b) Reduce the solubility of the drug by adding dextrose, sorbital, or any other excipients to produce a suspension of the drug. (c) Store the drug in a dry form and add water upon dispensing. If the label indicates “dry”, there is a desicant (e.g., silica gel), which absorbs physically “water” (polar molecule) from air when a cap is open (e.g., tetracycline granules). (d) Choose a proper buffer system if hydrolysis is caused by a general acid– base catalyzed hydrolysis process. (e) Incorporate a drug within micelles. (f) Complex a drug with some other excipients so that the complex is more stable (e.g., penicillin G procaine). (g) Keep the medication cold. |
|
|
|
Functional group susceptible to oxidation
|
Phenolic hydroxyl
<=>-OH Enolic hydroxyl - CH = C - OH Thiols - C – SH Sulfides - C - S - C - Ethers - C - O - C - Unsaturated olefin - CH2 - CH = CH - C - Aldehydes R- COH Amines (primary, secondary, and tertiary) |

|
|
|
Active drug in salt form
|

|
|
|
|
Elixirs
|
Clear, sweetened, hydroalcoholic solutions that are usually flavored to enhance their palatability and are suitable for drugs that are not soluble in water but soluble in water/alcohol mixtures.
he pharmacist must beware whether a patient takes drugs which possess an antabuse-like activity (e.g., metronidazole and chlorpropramide) |
|
|
|
Emulsions
|
An emulsion is a heterogeneous dispersion in which at least one immiscible liquid is dispersed in another liquid as small droplets or globules.
When two immiscible liquid phases (e.g., oil and water) are mixed, the small droplets formed increase the interfacial surface area and intend to coalesce to reduce surface free energy without continuous mechanical stirring and thus are thermodynamically unstable. In order to stabilize the integrity of individual droplets and prevent coalescence without continuous mixing, the third component (i.e., emulsifying agents) is employed to obtain pharmaceutical emulsions. Presently pharmaceutical uses of emulsions are limited to topical and injections. |
|
|
|
Bottle (Forbes Bottle) Method
|
Preparing primary emulsions
This is a variation of the dry gum method for volatile oils or oleaginous materials of low viscosity. The proportion of oil, water, and acacia gum is 3:2:1 or 2:2:1. Oil and acacia gum are placed in a dry bottle and the mixture is shaken.  Two parts of water are added in small increments and the mixture is thoroughly shaken after each addition (primary emulsion). The primary emulsion is diluted to the proper volume with water and other ingredients. |
|
|
|
Soap Method
|
Preparing primary emulsions
Equal volumes of an oil and water containing alkali (e.g., KOH, NaOH, NH4OH, sodium borate) or alkali earth [e.g., Mg(OH)2, Ca(OH)2] hydroxides are mixed. Free fatty acid present in the oil reacts with the hydroxide to form a soap, which acts as an emulsifying agent. Some oils do not have a sufficient amount of free fatty acid (e.g., cottonseed oil, peanut oil, and other vegetable oils) and thus the addition of a little excess of oil ensures a nice, homogenous emulsion. However, olive oil contains enough oleic acid. Lime and alkali soaps form W/O and O/W emulsions, respectively. Addition of an acid converts the carboxylate of the emulsifying soap into the carboxylic acid, which is not an emulsifying agent, causing the destruction of the emulsion. |
|
|
|
Oil - in - water emulsion
|
Water is in continuous phase
oil – internal phase and water – external phase |
|
|
|
Water - in - oil emulsion
|
water – internal phase and oil – external phase
|
|
|
|
74% rule of emulsions
|
You can add up to 74% oil in water to maintain oil - in - water emulsion.
However, above 74%, the emulsion turns into water - in - oil emulsion. Phase inversion |
|
|
|
Bancroft's rule
|
Mixture of 70% oil in 30% water shaken up results in water - in -oil emulsion
Addition of water soluble emulsifying agent to 70% oil and 30% water results in oil - in -water emulsion. Water is in continuous phase. However, if you have 75% oil and 25% water, addition of water soluble emulsifying agent will result in oil - in - water according to Bancroft's rule. This is not the case because the 74% rule overrides the Bancroft rule so you will have water - in - oil emulsion. |
|
|
|
Anionic pharmaceutical preservatives
|
Benzoic acid
Sorbic acid Thimerosal Phenylmercuric Acetate Sodium oleate, sodium palmitate, sodium lauryl sulfate, sodium dioctyl sulfosuccinate |
|
|
|
Neutral pharmaceutical preservatives
|
Methylparaben
Propylparaben Benzyl Alcohol Chlorobutanol Span 60 Tween 20 |
|
|
|
Cationic pharmaceutical preservative
|
Benzalkonium Chloride
Cetylpyridinium Chloride Benzethonium Chloride Hexadecyltrimethyl ammonium chloride |
|
|
|
Three types of emulsifying agents
|
Surface – Active Agents (Surfactants)
Hydrophilic Colloids Finely Divided Particles |
|
|
|
Hydrophilic colloids
|
Naturally occuring materials: composition and emulsion property vary batch to batch and are susceptible to bacterial growth. Therefore they are used extemporaneously in the preparation of O/W emulsions. Ex: polysaccharides (e.g., acacia, tragacanth, chondrus, alginates and pectin) and proteins (gelatin and casein).
Semi-synthetic cellulose derivatives: overcome the batch to batch variation: methylcellulose and sodium carboxymethylcellulose. Even though they do not lower appreciably the interfacial tension, firm multi-molecular films at the interface are formed to provide a good mechanical barrier and resist to rupture and prevent coalescence. Hydrophilic colloids increase the viscosity of aqueous phase and frequently used with surfactants to stabilize the emulsion. Some of these materials carry ionic charge, which provides electrostatic repulsion as an additional obstacle and thus prevents the coalescence of emulsions. |
|
|
|
Finely Divided Particles
|
They can wet both oil and water and form a particulate film at the interface.
The size of particles should be much smaller than the droplet sizes. O/W and W/O emulsions can be produced depending on the emulsification methods. Ex: bentonite, aluminum and magnesium hydroxides, Veegum (aluminum silicate), talc, and carbon black. |
|
|
|
Ampholytic pharmaceutical preservatives
|
Lecithin
n - dodecyldimethybetaine |
|
|
|
Water – soluble anti-oxidants part 2
|
Organic :
Ascorbic acid Thiourea Thioglycerol Thioglycolic acid |
|

