![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
272 Cards in this Set
- Front
- Back
|
Arabidopsis Genetic Nomenclature |
•Wild type allele of a gene; uppercase italics –LEAFY or LFY •Mutant alleles; lower case italics –leafy orlfy •Plants with mutant phenotype –leafyplants or leafymutants •Protein product of the gene uses the same name; uppercase plain text –LEAFY or LFY •Mutant proteins; lowercase plain text –leafy or lfy •Mutations that produce similar phenotypes often have similar names –leafy1, leafy2 and leafy3. •LEAFY1, LEAFY2and LEAFY3define three separate genes. •Mutant alleles of the same gene are designated by hyphenated numbers, for example leafy2-1 andleafy2-2. |
|
|
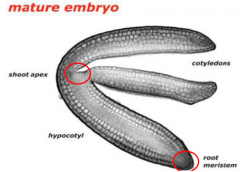
Embryogenesis |
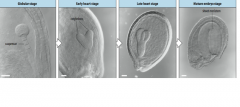
Embryogenesis covers development from the time of fertilization until dormancy occurs. 1. Establish the basic body plan. Radial patterningproduces three tissue systems, and axial patterningestablishes the apical-basal (shoot-root) axis. 2. Set aside meristematic tissuefor postembryonic elaboration of the body structure (leaves, roots, flowers, etc.). 3. Establish an accessible food reserve for the germinating embryo until it becomes autotrophic |
|
|
Ovule Structure |

f -funiculus oi-outer integument m -micropyle |
|
|
Mature Embryo Sac |
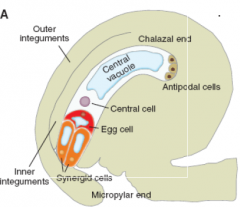
Note the polarity of each cell, i.e. the position of the cells within the sac and the position of the nucleus and vacuole within each cell. |
|
|
Stages of Arabidopsis embryo development |
-Egg -Two cell -Octant -Globular -Triangular -Heart -Torpedo -Mature embryo
|
|
|
Fate map: apical-basal axis |
Eight cell stage: Four anatomically distinct cell types are present: The upper (green) and lower (yellow-green) tiers of the proembryo, the hypophysis (purple) and the suspensor (grey). |
|
|
Fate map: radial axis -Zygote |
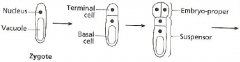
|
|
|
Fate map: radial axis -Globular embryo |
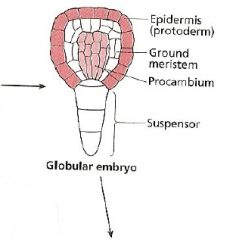
|
|
|
Fate map: radial axis -Heart-shaped embryo |
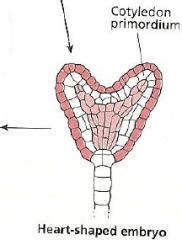
|
|
|
Fate map: radial axis -Torpedo-stage embryo |
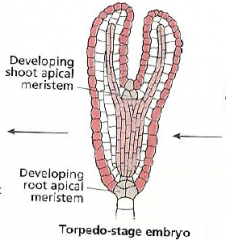
|
|
|
Fate map: radial axis -Mature seed |
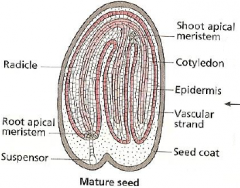
|
|
|
Seed maturation is characterized by: |
-accumulation of storage compounds -acquisition of dormancy -acquisition of desiccation tolerance |
|
|
Monocot |
-mature seed is largely composed of endosperm with a small embryo -Storage reserve usually starch e.g. wheat, maize |
|
|
Dicot |
-mature seed is largely the embryo with perhaps a remnant layer of endosperm Storage reserve usually protein (pea) or oil (brassica, sunflower) |
|
|
Seed structure (dicot) |
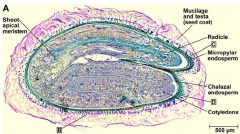
|
|
|
Endospermic |
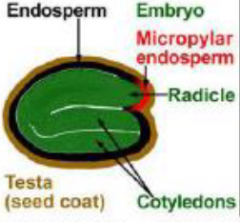
|
|
|
Non-endospermic |
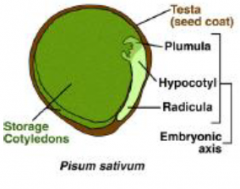
|
|
|
Perispermic |
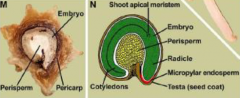
|
|
|
Seed structure (monocot) |
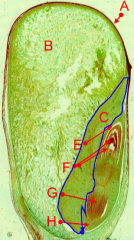
A = pericarp (fruit wall) fused with testa (seed coat) B = endosperm C = scutellum (or cotyledon) E = coleoptile (i.e. protective sheath of the plumule) F = plumule (embryonic shoot) G = radicle (embryonic root) H = coleorhiza (protective sheath of the radicle) |
|
|
Endosperm function |
•Required for embryogenesis and seed germination (nurse tissue –placenta) •Acts as an intermediary between the embryo and surrounding maternal sporophyte. •Post-fertilization barrier. |
|
|
Endosperm development |
•Life cycle is confined to the seed stage •Different modes of development (e.g. nuclear/helobial/cellular-type) |
|
|
Endosperm Development: free-nuclear phase |
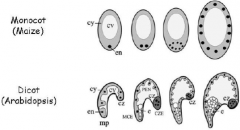
|
|
|
Coenocyte |
-a cell with multiple nuclei in the same cytoplasm |
|
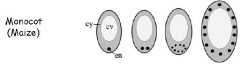
|
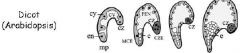
The triploid endosperm nucleus (en) is located in the basal cytoplasm of the central cell. A large central vacuole (cv) fills up most of the volume, surrounded by a thin line of cytoplasm (cy). The central cell nucleus divides without the formation of cell wall between sister nuclei. After three rounds of nuclear divisions, eight endosperm nuclei are located in the basal endosperm coenocyte. At the end of this phase the complete endosperm coenocyte contains evenly spaced nuclei in the entire cytoplasm. |
|
|
The Arabidopsis endosperm coenocyte has nuclei migrating from the micropylarregion (mp) toward the chalazalend (cz), eventually covering the entire periphery of the coenocyte. As development progresses, the endosperm coenocyte develops three distinct regions: the region surrounding the embryo (MCE), the central or peripheral endosperm (PEN), and the region of the chalazalendosperm (CZE), which contains the chalazalcyst (cz). At the end of the globular embryo stage, the embryo becomes completely surrounded by MCE cytoplasm. |
|
|
Endosperm Development: cellularization |
•Initiated by the formation of radial microtubule system on nuclear surfaces. •Microtubules from neighboring nuclei eventually meet, forming interzonesin which wall material is deposited. |
|
|
Endosperm balance number |
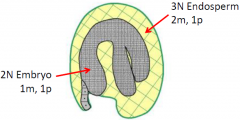
•Proper balance of maternal (m) and paternal (p) genomes are needed for development. •Endosperm ploidy ratio: Cross two diploids -> 2m:1p -> normal endosperm development Cross two tetraploids -> 4m:2p -> normal endosperm development Cross tetraploid (female) with diploid (male) -> 4m:1p -> aborted seed(unbalanced endosperm genome ratio) |
|
|
Genomic imprinting regulates endosperm development |
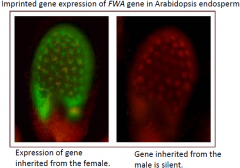
•Maternal-and paternal-specific gene expression in the endosperm. |
|
|
Examples of plant hormones |
-auxin (IAA) -cytokinins -gibberellins (GA) -Abscisic Acid (ABA) -Ethylene -Brassinosteroids -Salicylates -Strigolactones -Jasmonates |
|
|
Plant vs. animal hormones |
Plants Animals Number of hormones fewer many Specificity of action non-specific specific Work together yes no Specific production site no yes Specific site of action no yes
Fewer plant hormones, each elicits a variety of responses and often works together with other hormones (cross talk). |
|
|
Mode of action |
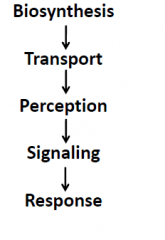
Biological response is determined by …. 1.Hormone concentration. 2.Ability of target tissue/cell to recognize the hormone (concentration of receptor). 3.Condition of the plant ( e.g. plant age/developmental stage) Site of action x developmental stage x concentration of hormone = response |
|
|
Regulation of plant hormone levels |
Many tightly regulated biochemical pathways contribute to active hormone accumulation. Conjugation can temporarily store a hormone in an inert form, lead to catabolic breakdown, or be the means for producing the active hormone. |
|
|
Hormone Transport |
Hormones can move: •through the xylem or phloem (transport tissue) Xylem: ABA, GA and cytokinins Phloem: auxin, GA, ABA and cytokinins Hormones can move: •across cellular membranes •through regulated transport proteins |
|
|
Hormone perception |
-Receptors can be membrane-bound -Hormone binding initiates an information relay -Soluble receptors can facilitate interactions between proteins -Hormones can act like “molecular glue” |
|
|
Hormones and protein ubiquitination |
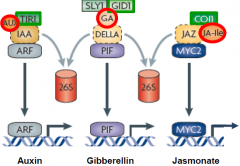
The hormones (red) bind to receptors (green), initiating proteolysis of repressors (yellow) to activate a transcriptional regulator (blue) |
|
|
Abscisic Acid (ABA) |
• A single compound. • Discovered as a inhibitor of auxin from plant extracts. • Synthesis occurs in most tissues, especially leaves and seeds. • ABA synthesis is tightly regulated. Synthesis strongly induced in response to stress. • Regulate seed maturation and dormancy, desiccation tolerance, stress response and stomatal aperture. |
|
|
ABA in plant development |
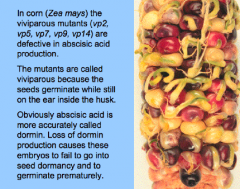
• Growth Inhibitor (e.g. inhibits root growth, cotyledon greening and expansion). • Promotes seed maturation and dormancy. • Prevents vivipary (development of the embryo without a dormant period).
|
|
|
Gibberellins (GA) |
• More than 110 different gibberellins are known - abbreviated GA1...GAn. • No more than 12 or so different gibberellins occur in any one species. • Only a few of the GA's have biological activity. GA3 is the most active in Arabidopsis. • Synthesis occurs in young leaves, roots, and developing seeds and fruits. • Transport occurs through xylem, phloem, or cell-to-cell. Phloem seems to be most important transport route. • Regulate growth (e.g. leaf expansion and stem elongation), promote seed germination and promote flowering. |
|
|
How do gibberellins regulate growth? |
The pea mutant le, studied by Mendel, encodes GA3 oxidase, which produces active GA. Loss of function of le reduces active GA levels and makes plants dwarfed. Semidwarf rice varieties underproduce GA because of a mutation in a the GA20 oxidase biosynthetic gene. “Green revolution” gene. |
|
|
Ethylene |
• Single compound. • A gaseous plant hormone. • Made by all parts of the plant. Meristematic regions (shoot apex) and senescing tissues are rich sources. • Production is stimulated during physiological stresses (wounding, flooding, drought), senescence and ripening. • Cell division and elongation; fruit ripening; flowering; senescence (leaf and petal) ; root growth; epinasty (downward bending of leaves); triple response; thigmomorphogenesis (change in growth form in response to touch); promotes germination. |
|
|
Ethylene perception mutants interfere with signalling |
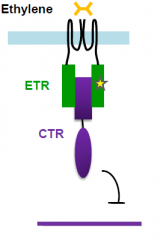
Mutations that affect ethylene perception and signaling interfere with triple response. etr mutant fail to undergo triple response in the presence of ethylene ctr mutant undergoes triple response in the absence of ethylene |
|
|
Crosstalk between hormone signaling pathways |
Crosstalk (or cross-regulation) occurs when two pathways are not independent. It can be positive and additive or synergistic, or negative. Crosstalk can affect the synthesis, transport or signaling pathway of another hormone. |
|
|
Seed germination |
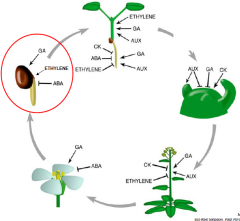
Events initiated upon imbibition of non-dormant seed and leading to radicle protrusion through the seed external envelopes (testa and endosperm).
|
|
|
ABA and GA during germination |
Induction and maintenance of seed dormancy by abscisic acid (ABA) and dormancy release by gibberellin (GA). ABA mutant - seeds germinate prematurely. |
|
|
Auxin |
• Five naturally occurring auxins isolated from plants. Indole-3-acetic acid (IAA) is the most abundant. •Regulate -Growth -Phototropism and gravitropism -Branching -Embryonic patterning -Stem cell maintenance -Organ initiation Charles Darwin studied the way seedlings bend towards light, a direct effect of auxin action. Darwin concluded that a signal moves through the plant controlling growth. Differential cell growth is a result of auxin movement to the shaded side.
|
|
|
Polar auxin transport |
•Auxin is made in actively growing tissue which includes young leaves, fruits, and especially the shoot apex. •Auxin is transported in a polar manner throughout the plant. Promote the formation of local auxin maxima/gradient. •Auxin is protonated (IAAH) in the acidic extracellular space (pH 5.5) and enters the cell via diffusion or an influx carrier, AUX1 (blue). •In the cytoplasm (pH 7), auxin is deprotonated (IAA-) and can only exit the cell via auxin efflux carrier, PINFORMED (PIN; red). • Asymmetric distribution of the transporters controls polar auxin transport. |
|
|
Auxin regulates plant development |

-Lateral organ initiation at the shoot apical meristem -Patterning and vascular development -Maintain stem cell fate at the root apical meristem -Embryonic patterning -Inhibit branching in the shoot -Promote branching in the root |
|
|
Cytokinin |
•Family of related adenine-like compounds. Most common form is zeatin which was isolated from corn. •Synthesized primarily in the meristematic region of the roots. Also produced in developing embryos. •Root-produced cytokinins are transported acropetally, via xylem, to the shoot tip. •Regulate cell division, control of leaf senescence, control of nutrient allocation, root nodule development, stem cell maintenance, and regulate auxin action. |
|
|
Cytokinins affect grain production and drought tolerance |
Rice plants that accumulate more CK can produce more grain per plant because of changes in inflorescence architecture. Tobacco plants that produce more CK are more drought tolerant because of the delay in leaf senescence conferred by CK. |
|
|
Cytokinin act antagonistically to auxin |
CK: Promote stem cell fate at the shoot apical meristem Promote branching in the shoot Inhibit branching in the shoot Promote differentiation at the root apical meristem Auxin: Promote lateral organ initiation at the shoot apical meristem Maintain stem cell fate at the root apical meristem. Inhibit branching in the shoot Promote branching in the root |
|
|
Role of cytokinin and auxin in plant regeneration |
High cytokinin Low auxin callus forms shoots
Intermedate levels of cytokinin + auxin Undifferentiated callus
No cytokinin No auxin Control, no callus
Low cytokinin High auxin Callus forms roots |
|
|
Embryogenesis |
Establish the basic body plan. Radial patterningproduces three tissue systems, and axial patterningestablishes the apical-basal (shoot-root) axis. |
|
|
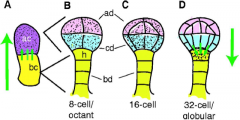
Apical-basal pattern formation |
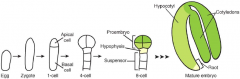
Zygote expands and divides asymmetrically to produce the apical and basal cells. Asymmetric division…. -establishes apical-basal polarity of the embryo -establishes two cell lineages –apical (embryo) and basal (extraembryonicsupport structure, suspensor). Embryo stages are based on the number of cells in the apical domain only, thus the first zygotic division results in a one-cell stage embryo. |
|
|
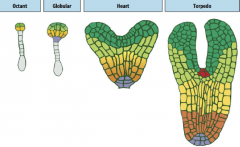
Invariant cell division pattern |
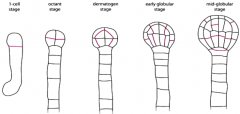
•Asymmetry must occur followed by division in each lineage. -The apical cell undergoes a series of longitudinal and transverse divisions to generate the eight cell proembryo. -The basal cell undergoes a series of strictly transverse divisions to generate the suspensor. Asymmetry must occur to produce to root meristem. Uppermost cell of the suspensor, hypophysis, becomes part of the embryo. At the mid-globular stage, the hypophysisundergoes an asymmetric division to yield a smaller lens-shaped cell and a larger basal cell, the precursors for cells within the root meristem. |
|
|
Intrinsic and extrinsic cell fates |
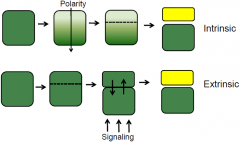
Asymmetric division produce cells of different shape and size (morphology) and fates. Two mechanism to produce cells of different fates: intrinsic and extrinsic. |
|
|
YODA (YDA) |
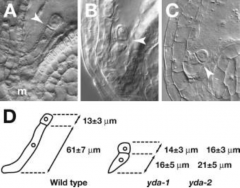
Required for zygote elongation and asymmetric division YODA is required for specification of suspensor cell fate. |
|
|
Aberrant division patterns in the basal lineage of yda mutants |
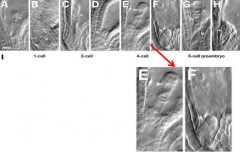
wild-type embryos: A, C, E, and G ydamutant embryos: B, D, F, and H |
|
|
YODA is required for specification of suspensor cell fate. |
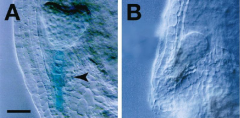
A: •Wild type heart stage •Expression of suspensor specific gene
B: •ydamutant heart stage •NO expression of suspensor specific gene |
|
|
Pattern formation in yda mutant embryos |
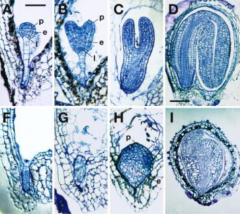
A-D: Wild type F-I: YODAmutant p –protoderm e –vasculature l -hypophysis |
|
|
What provides the cue for asymmetry and specification of the first embryonic cell fate decision? |
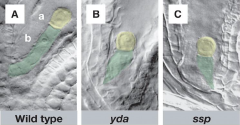
Pollen provides patterning cue!
|
|
|
SHORT SUSPENSOR (SSP) |
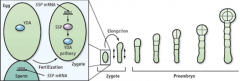
Required for zygote elongation and asymmetric cell division. Mutation affects development only if its paternally derived. SSP transcript is made in sperm cells. SSP protein (green) is detected in developing zygote. •SHORT SUSPENSOR (SSP)transcripts delivered to the egg by the pollen sperm cell. •Trigger zygote elongation and the first asymmetric cell division after fertilization. •Activates YODA (YDA) dependent signaling. •Establishes apical-basal polarity of the embryo.
|
|
|
Does polarity of the ovule/embryo sac determine polarity of the zygote? |
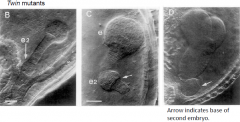
•Multiple embryos of the same zygote can develop inverted apical-basal axis. •Uncoupling of apical-basal pattern formation from polarity of ovule. |
|
|
What regulates cell division and cell fate specification in the apical and basal cell lineage? |
•WUSCHEL-related homeobox(WOX) transcription factors Homeoboxgenes that encode for transcription factors. •Regulators of cell division and cell fate specification. WOX2: required for apical cell lineage specification. WOX8andWOX9: required for basal and apical cell lineage specification. |
|
|
Apical-Basal Pattern Formation |
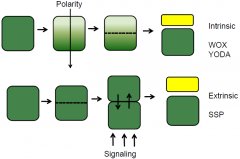
Embryo pattern formation require a combination of both intrinsic and extrinsic factors. |
|
|
Monopteros(mp) |
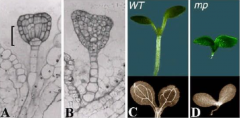
Defectivein embryonic auxinsignaling, fail to form an embryonic root meristem and therefore yield rootless seedlings. |
|
|
Auxin transport in the developing embryo |
Green arrows indicate the direction of auxin transport; stippling indicates regions with high auxin levels. •A flux of auxin is established that first directs auxin towards the apical cell and then switches to direct auxin away from the apical cell towards the embryonic base. •Polarity of this second flux determines the embryonic shoot–root polarity that remains with the plant for the rest of its life. Basal‘auxin maximum’ in the hypophysisis required for root formation. |
|
|
Model for auxin transport and action in the plant cell. |
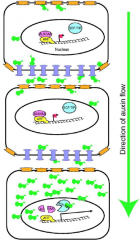
Monopteros (MP) is an auxin response factor (ARF). ARF = transcription factor AUX/IAA = transcriptional repressor SCF-TIR = E3 ubiquitin ligase
|
|
|
Auxin plays a pivotal role in establishing apical-basal polarity. |
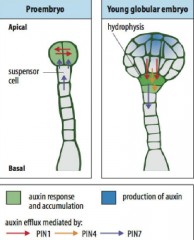
•Initially, auxin funnelled toward the developing proembryoby PIN7, which accumulates in the apical membrane of the basal cell and its descendents. •PIN1, which is expressed in the proembryo, does not show polar distribution at first but becomes preferentially localized to the basal membrane of the provascularcells (the central cells of the lower tier) by the early globular stage, establishing apical–basal auxin flux. |
|
|
Radial pattern |
The radial pattern is arranged in three concentric layers of tissues: the outer protoderm, the inner mass of ground tissue, and the centrally located procambium. |
|
|
Stereotypical pattern of cell divisions produces each tissue type |
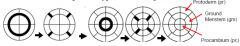
Protoderm, ground meristem, and procambium are separated as the progenitors of the major tissue types in the embryonic axis by regular periclinal and anticlinal divisions. •Procambium cells (pc) perform periclinal division to generate the pericycle (p) and vascular (v) primordium (xylem and pholem). •Asymmetric periclinal divisions divide the ground meristem (gm) into cortex (c) and endoderm (ed). •Protoderm cells divides anticlinally to increase the number of cells in the epidermis (ep). |
|
|
Radial axis root |
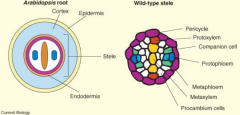
|
|
|
Radial Pattern Formation |

Depends on the two GRAS family transcription factors SHORTROOT (SHR) and SCARECROW (SCR). |
|
|
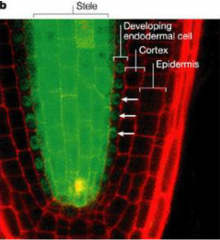
SHORT-ROOT (SHR) and SCARECROW (SCR) expression in the radicle (embryonic root) |
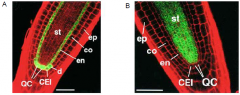
SCARECROW is required for asymmetric cell division A. SCARECROW promoter driving GFP expression in the Arabidopsis root meristem. B. SHORTROOT promoter driving GFP expression in the Arabidopsis root meristem.
ep=epidermis, co=cortex, en=endodermis, CEI=cortex/endodermis initial, QC=quiescent centre, st=stele. |
|
|
SHOORTROOT moves across cell layers |
SHORTROOT promoter driving expression of a SHORTROOT-GFP fusion protein. |
|
|
SHORTROOT is responsible for specifying endodermis |
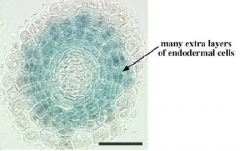
Ectopic expression of the SHORTROOT |
|
|
Endodermal cell specification |
Signal for endodermal cell specification originates from the stele. SHOORTROOT (SHR) and SCARECROW (SCR) are required for cell division and endodermal cell specification. |
|
|
knolle Mutants |
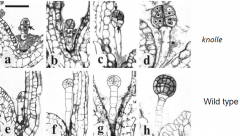
•Cell division is abnormal; cytokinesis is disrupted (arrows). •Multinucleate cells (asterisks). •Protoderm (epidermis) formation is disrupted. knolle mutant seedlings…. ..lack a well-formed epidermis layer. ..lack functional meristems. ..die after germination. Knolle gene mutation disrupt the regular pattern of embryogenesis by altering the rate and plane of cell division. •Knolle gene is expressed in mitotically dividing cells; protein localizes to the cell plate. •Knolle is required for cell plate formation; mediates vesicle trafficking and fusion. |
|
|
keule mutants |
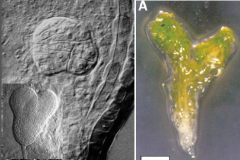
•Mutant keule embryos have large multinucleate cells with gapped or incomplete cell walls. •Cell division appears to be slowed down, and the planes of cell division are often disoriented. •Morphology of the protoderm (epidermis) cells is affected. •keule mutants die after germination. KNOLLE and KEULE genes interact to promote vesicle fusion during cytokinesis. |
|
|
fass mutant and fate cell specification |
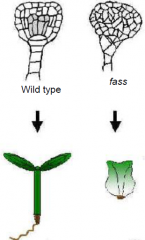
•Distort cell divisions result in irregular cell patterns. •Radial and longitudinal patterns of tissue and organs are preserved.•Fass mutants show an inability to form microtubule preprophase bands. •FASS gene product is required for the accurate positioning of cell walls during division. |
|
|
fass plants |
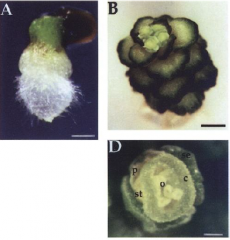
In fass mutants the regular sequence of cell divisions is dramatically disturbed. Disorganisation of normally precise cell division patterns during embryogenesis does not necessarily perturb cell fate patterning. Specification of the basic embryonic pattern is largely, if not entirely, based on positional cues, rather than on cell lineage.• miniature, every organ present i n reduced size. •A. Mutant seedling at 5 day old, showing root, hypocotyl, and shoot. •B. Mutant plant at 58 day old, showing rosette and flowers. •D. Mutant flower of a 57 day old plant. Floral parts: se, sepals; p, petals; st, stamen; c, carpels; o, ovules. |
|
|
Primary development: stem |
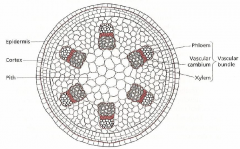
The dicot internode after primary development (radial cross-section) |
|
|
Primary development: root |
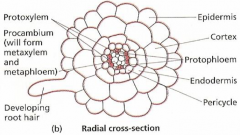
|
|
|
Primary development: leaf |
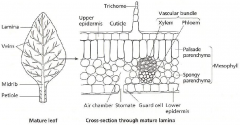
|
|
|
Secondary development includes... |
...Cork and phelloderm from the protoderm Fasicular cambium (from the provascular tissue) and Interfasicular cambium (from the ground meristem) -These two things together lead to the vascular cambium, which leads to the secondary xylem, and the secondary phloem, which leads to the cork cambium. |
|
|
Secondary development: shoot |
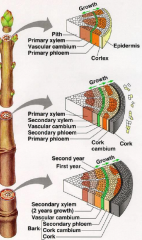
The vascular cambium between the primary xylem and phloem remain meristematic and produces secondary xylem towards the inside & secondary phloem towards the outside. This produces concentric rings of secondary vascular tissues with the vascular cambium between them. |
|
|
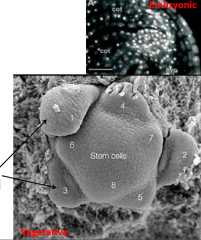
Meristems |
•Meristems are organized cellular structures capable of indeterminate growth. •Each contains core of undifferentiated “stem” cells which can divide and differentiate to produce adult tissues, while maintaining and regenerating the meristem. •Meristems are self-organizing, for example, meristems regenerate after bisection. • Meristems activity is often iterative. Primary development relies on meristems that were made during embryogenesis. All postembryonic structures of the plant are generated from these small collections of pluripotent cells. |
|
|
Different types of meristems |
• Root Apical Meristem (RAM) • Shoot Apical Meristem (SAM) • Inflorescence meristem (IF) • Auxiliary meristem (AM) • Floral Meristem (FM) |
|
|
Shoot Meristems |
• Entire shoot system is derived from post-embryonic development in the shoot apical mersitem (SAM). • Growth occurs by the production of primordia which develop at the meristem periphery. • Vegetative SAMs are indeterminate, and in some species are capable of growth for thousands of years. • After a phase of vegetative growth, the shoot apex changes to become an inflorescence meristem, which in turn produces many floral meristems (determinate) that produces flowers. |
|
|
Primordia |
Growth occurs by the production of primordia which develop at the meristem periphery. Primordia (singular – primordium) undergo cell division and differentiation to develop into structures such as leaves or into additional meristems. |
|
|
Vegetative development in shoot apical meristems |
|
|
|
Inflorescence development in shoot apical meristems |
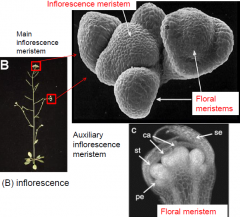
|
|
|
Meristems activity is iterative |
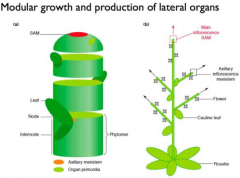
The activity of meristem determine plant architecture. A vegetative meristem will produce modular units (phytomers) each consisting of a leaf, bud, and internode. |
|
|
Auxillary meristems |
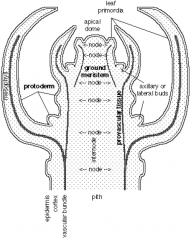
Vegetative meristem produce modular units (phytomers) consisting of a leaf, bud, and internode. Auxuillay or lateral buds • Forms at a node between the leaf primordium and the stem. • Contains a nest of cells that keep a meristematic character. Vegetative meristem produce modular units (phytomers) consisting of a leaf, bud, and internode. • Vegetative auxiliary meristems have the potential to develop into lateral shoots. • Lateral shoots reiterate the pattern of development in the primary shoot. • Also have the alternative option of remaining quiescent as an auxiliary bud. • Outgrowth of auxiliary buds into active shoot is regulated by endogenous and environmental cues. |
|
|
Organization of the shoot apical meristem (SAM) |
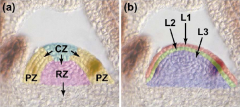
Meristem is organized in to functional zones (a) and layers (b). central zone – CZ; peripheral zone – PZ; rib zone - RZ |
|
|
Meristem functional zones |
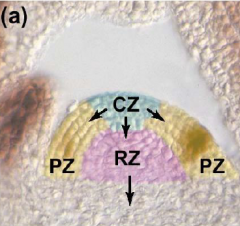
• Central zone (CZ) - very slowly dividing. Contains a core of stem cells. Allocates cells to peripheral zone and rib zone • Peripheral zone (PZ) - slightly more rapidly dividing. Site of organ initiation (e.g. leaf primordia). • Rib zone (RZ) - allocates cells to the stem.
|
|
|
Meristem functional layers |
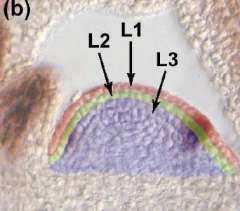
• L1- a single layer of cells that gives rise to the epidermis. • L2- a single layer that gives rise to ground tissue. • L3 (corpus cells) - forms the body of new tissues, including vasculature. • L1 and L2 together are called the tunica. |
|
|
Maintaining the SAM |
Meristem must… •promote cell division to replenish cells lost to differentiation. •restrict cell division to prevent meristem overgrowth. Plants must maintain this homeostasis throughout their life span, sometimes hundreds of years. |
|
|
Shoot apical meristem mutants |

|
|
|
clavata (clv) |
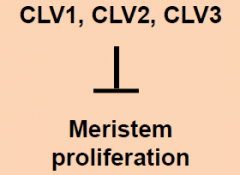
Mutants possess enlarged shoot meristems. • clavata mutants show a loss of control of meristem cell proliferation. • Three loci: CLAVATA1, CLAVATA2, CLAVATA3: three separate genes, all with similar phenotypes. |
|
|
Model for CLAVATA genes function |
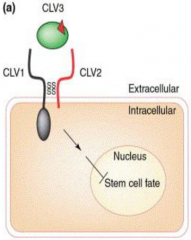
•CLAVATA1 (CLV1) - receptor kinase protein. •CLAVATA2 (CLV2) - receptor-like protein partner for CLAVATA1. •CLAVATA3 (CLV3) -a secreted peptide ligand for the CLV1/CLV2 receptor complex. •CLV1 and CLV2 (receptor complex) acts with CLV3 (ligand) to control the balance between meristem cell proliferation and differentiation (fate specification). |
|
|
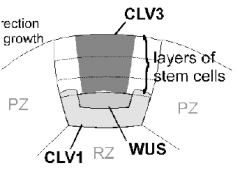
CLAVATA expression domains |
|
|
|
WUSCHEL (wus) |
• WUSCHEL (wus) mutants fail to form a shoot meristem in the embryo, but adventitious meristems form later. •“Rescued” meristems continually form and abort. •WUSCHEL is required to maintain the SAM meristem. •WUS encodes a homeodomain transcription factor. •Expression is limited to a small domain of cells deep within the meristem. •WUS expression is inhibited by the activity of the CLV complex. •WUS expression is greatly expanded in clv mutants (CLV pathway necessary to restrict WUS expression and cell division) •WUS expression is downregulated in CLV3 overexpressors |
|
|
Model for CLAVATA genes function |
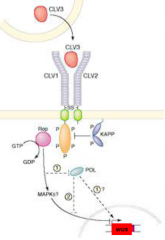
•WUS is a target for CLV signal transduction. •CLV signaling inhibit WUS gene expression. |
|
|
Maintaining the SAM |
CLV activity restricts size of stem cell population. -loss of CLV, increase SAM size WUS promotes division of stem cell. -loss of WUS, no SAM
|
|
|
Fate of stem cells is regulated by negative feedback. |
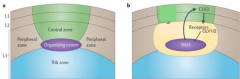
WUS and CLV comprise a regulatory loop, such that WUS promotes CLV3 activity, while the CLV complex negatively regulates WUS. When meristem gets too small - number of CLV1/ CLV3 expressing cells goes down, WUS is derepressed and activates cell division, increase in SAM. When meristem gets too big - number of CLV1/CLV3 expressing cells goes up, repressing WUS and repressing cell division, decrease in SAM. |
|
|
Hormonal regulation of SAM activity |
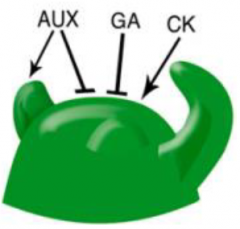
• Accumulation of auxin triggers organogenesis (differentiation) at the periphery of the SAM. • Cytokinin (CK) is essential for maintaining meristem activity. CK deficiency reduces SAM size and activity. CK stimulates the expression of genes involved in GA catabolism to reinforce the low-GA levels. |
|
|
shoot meristemless (stm) |

Required for maintenance of undifferentiated cells in Arabidopsis shoot meristems. STM expression: first observed in a few apical cells at the late globular stage embryo – the future SAM. Expressed throughout the SAM (blue) but not in organ primordia (arrows). |
|
|
Hormonal regulation of SAM activity |
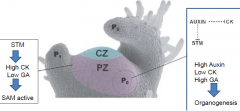
Shoot Meristemless (STM) controls the balance between the two hormones in the SAM by inducing cytokinin production (high CK), directly inhibiting GA synthesis, and indirectly promoting GA breakdown (low GA). |
|
|
Root Zones |

Developmental zones of the Arabidopsis root Differentiation zone •Root hairs begin to develop Elongation zone Meristematic zone • Small cells • Covered by a root cap Different types of cell expansion occur in different regions of the root. |
|
|
Root organization |
Vascular Bundle: 2 protoxylem elements, 2 protophloem elements.
Pericycle: 12 cells on average, source of lateral root Initiation, cells closest to xylem form lateral roots.
Endodermis and cortical cells: each forms a ring of exactly 8 cells.
Epidermis: surrounds the cortical cells. 2 types - those touching two cortical cells will form root hairs, those that touch one will not.
Lateral root cap and columella cells: protects the root as it penetrates soil. Columella cells have amyloplasts—required for gravity response. |
|
|
Root meristem |
Meristem: Quiescent Center (QC) and Initials QC • 4 cells in Arabidopsis • Surrounded by initials Initials • Stem cells that divide to give a rise to root tissue. • Each cell file originates from an initial cell. |
|
|
Cell division in the root meristem |
Root tissues are produced from root apical meristems by a highly sterotypical pattern of cell divisions. • Initials divide to give a new initial and a daughter cell. • Daughter cells divide further and differentiate. Quiescent center cells divide infrequently. Cell division rates increase progressively up the root. Frequency of observed cell division is represented by different densities of stippling. |
|
|
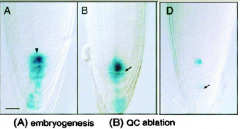
Quiescent center function |
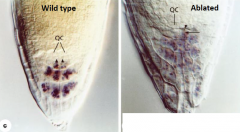
Ablation of a single QC cell (arrow) results in cessation of division of the contacting columella initial. Ablation of a single QC cell (arrowhead) triggers the differentiation of the neighboring columella initial (arrow). • Inhibit differentiation of neighboring cells so as to maintain the surrounding initial cells as stem cells. • Differentiation occurs within the range of a single cell of QC. • Stem cell reservoir- ensures regeneration and persistence of meristematic initial cells if they are lost. |
|
|
When quiescent cell is ablated |
1)abutting cortical initial cell divides longitudinally, acting like its daughter should. 2)neighboring columella differentiates. Conclusion - QC cells prevent initials from differentiating, not from dividing. |
|
|
Root meristem specification |
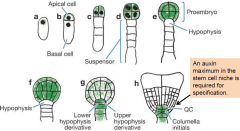
Auxin is required for specification of the root apical meristem. An auxin maximum in the stem cell niche is required for specification. Green – auxin accumulation |
|
|
Auxin transport and maxima: embryo |
•Initially, auxin funnelled toward the developing proembryo by PIN7, which accumulates in the apical membrane of the basal cell and its descendents. •PIN1, which is expressed in the proembryo, becomes preferentially localized to the basal membrane of the provascular cells (the central cells of the lower tier) by the early globular stage, establishing apical–basal auxin flux. |
|
|
Root meristem specification |
•Polar auxin-transport concentrates auxin at the basal pole of the embryo (hypophysis), leading to root formation. •Auxin regulates the expression of genes required to establish the root apical meristem, eg PLETHORA (PTL) genes. |
|
|
Auxin transport and maxima: root |
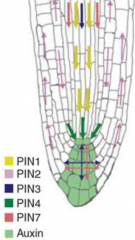
Auxin maxima in the root tip Expression of the auxin reporter DR5::GUS in columella cells, columella initial cells and QC. |
|
|
Root meristem specification |
|
|
|
QC cell-fate identity marker |
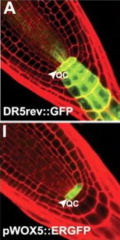
Wuschel-related Homeobox 5 (WOX5) •Similar to WUS in the shoot apical meristem •Required for specification and maintenance of QC cells |
|
|
Changes in expression of cell-fate identity marker, WOX5, after QC ablation. |
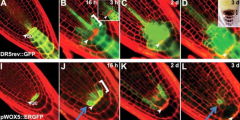
J: Expression of WOX5 in 1 to 2 cell layers proximal to the ablated QC. L: Expression of WOX5 gradually confined to central former provascular cells. |
|
|
Root meristem maintenance |
Auxin-cytokinin cross-talk controls root meristem size in Arabidopsis. • Repression of auxin activity by cytokinin enables differentiation of root cells. • Repression of cytokinin activity by auxin is essential for specifying and maintaining stem cell identity. |
|
|
SAM vs RAM |
• Number of cells RAM has fewer meristem cells • Cellular organization In SAM, discrete layers In RAM, cell files • Location In SAM, meristem is at the apex. In RAM, meristem is found subterminally (root cap is terminal structure). • Cell lineage In SAM, less strict correlation between meristematic cells and differentiated cell types. In RAM, cell lineage is easily defined. • Organizing centers RAM quiescent center and SAM o organizing center is necessary to promote the undifferentiated state of neighboring cells (stem cells). RAM quiescent center cells are stable, rarely divides. SAM organizing center cells are replaced continuously. RAM stem cells are a single layer abutting the quiescent center. |
|
|
Auxin and cytokinin in shoot apical meristem (SAM) |
Auxin: Promote lateral organ initiation at the shoot apical meristem
Cytokinin: Promote stem cell fate at the shoot apical meristem |
|
|
Auxin and cytokinin in root apical meristem (RAM) |
Auxin: Maintain stem cell fate at the root apical meristem
Cytokinin: Promote differentiation at the root apical meristem |
|
|
Apical Dominance |
• Outgrowth of auxiliary buds into active shoot is regulated by endogenous and environmental cues. • Shoot apex inhibits branching (auxiliary bud outgrowth) – Apical Dominance • Inhibitor of bud outgrowth originates from the shoot apex. |
|
|
Agar block |
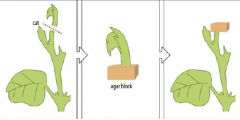
When the upper part of a sheet is removed the lateral buds beneath the cut start to grow. If the main apex is removed and placed on an agar block, auxin diffuses into the block, which can then inhibit lateral bud growth. |
|
|
Two hormones that regulate branching |
Auxin and cytokinin. They're antagonistic, so if one is growing, one is inhibiting. • Auxin inhibits auxiliary bud outgrowth. • Root produced cytokinin promote auxiliary bud outgrowth when transported to the shoot. • To maintain apical dominace shoot produced auxin inhibit the effects of cytokinin from the roots. |
|
|
Positioning of lateral organs on the SAM |
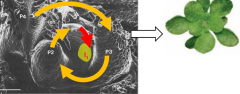
The vegetative shoot apical meristem give rise to primordia (P) in very regular and predictable temporal and spatial patterns. Spiral arrangement is characterized by a divergence angle between the organs of 137.5°, the golden angle. Leaf primordia are numbered P1, P2 etc from youngest to oldest. The next leaf to form is called the incipient primordium, I1. |
|
|
Phyllotaxis |

Arrangement of leaves (or other organs) on the stem and in relation to each other. |
|
|
Auxin and organ primordia initiation |
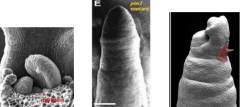
1: Wildtype Arabidopsis shoot apex 2: pin1 mutant shoot apex – fails to produce lateral organs. 3: Local auxin application (red blob) triggers organogenesis in the pin1 mutant. |
|
|
Local maxima of auxin is required to trigger primordia initiation |
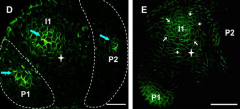
The expression and orientation of PIN was monitored in the shoot apex of plants expressing PIN1::PIN1-GFP. Leaf initiation is preceded by changes in the distribution of PIN1, contributing to a local auxin maximum. |
|
|
Auxin treatment caused PIN1 upregulation and ectopic primordium induction |
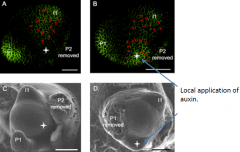
PIN1 distribution is dynamic during organogenesis. Green=PIN1 protein. Red arrows =orientation of direction of auxin flow. |
|
|
Model for auxin transport in the shoot apical meristem |
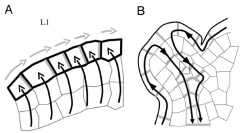
A: - AUX1 (black lines) help to concentrate auxin in the L1 surface layer (black arrows). - PIN1 (gray lines) direct auxin fluxes (gray arrows). - Creates auxin maxima. - Induce primordium initiation. B: After primordium initiation, PIN1 distribution changes, directing auxin flow into the developing veins of the leaf. Young organ is now an auxin sink. |
|
|
Model for auxin transport and primordia initiation |
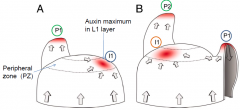
•Polarized localization of PIN1 leads to auxin accumulation primordia initiation (I1). •Basipteal transport in the primordia (P1) drains auxin into the inner layers, creating a sink, which depletes its surroundings from auxin and prevents the formation of new primordia within its vicinity. •Another auxin maximum (I1)is created in the PZ away from the developing P1. •Organ initiation at the shoot apical meristem is determined by auxin distribution and PIN1. •An auxin maximum is necessary and sufficient to specify the site of primordium formation. •Developing primordia affect auxin distribution and therefore placement of incipient primordia. Auxin distribution and redistribution patterns the formation of successive leaves with regular spacing. |
|
|
Auxin and organ primordia initiation |
Polar auxin transport is required for organogenesis in monocots and dicots and in plant with spiral and alternating patterns of phyllotaxy. |
|
|
Lateral root development |
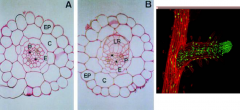
Lateral roots originate from pericycle cells of the primary root. Founder cells in the pericycle (P) undergo transformation and organize into lateral root primordia (LR), which later grow through the endodermis (E), cortex (C) and epidermis (EP) cell layers to emerge from the primary root. |
|
|
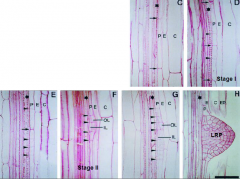
Invariant pattern of cell division produces a lateral root primordium (LRP) |
•Stage I. Founder pericycle (P) cell(s) undergoes a series of anticlinal divisions. •Stage II. Periclinal divisionproduces two layers, outer layer (OL) and inner layer (IL). •Stage III-VII. Further anticlinal and periclinal divisions create a dome-shaped primordium. |
|
|
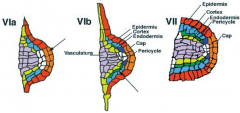
Lateral root organization |
All the radial pattern elements of the primary root are present in the lateral root primordium. |
|
|
Stages of lateral root development |

|
|
|
Lateral root initiation |
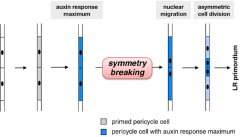
Lateral roots initiate at regular intervals along the primary root from pericycle cells adjacent to the xylem pole. • Auxin acts as a local morphogenetic trigger to specify lateral root founder cells. •Accumulation of auxin in xylem pericycle cells is a necessary and sufficient signal to respecify these cells into lateral root founder cells. |
|
|
Auxin and lateral root initiation |

•Auxin accumulation is a local morphogenic trigger for respecification of pericycle cells into lateral root founder cells. Lateral root initiation is induced by root curvature. AUX1-YFP accumulates uniformly in the pericycle cells on the outside of the curve prior to lateral root initiation. (A) 230 min before, (B) 90 min before, (C) 10 min after, and (D) 520 min after the first asymmetric cell division. Left shows a blowup of (A); right, a blowup of (C). White asterisks indicate the pericycle cell files. |
|
|
Auxin and lateral root development |
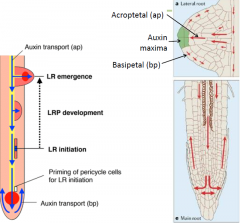
• Proper auxin transport is required for lateral root development.
|
|
|
Auxin and lateral root development in wild type versus pin protein |
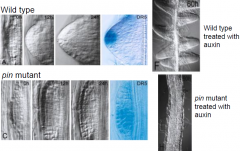
|
|
|
Auxin and lateral root emergence |
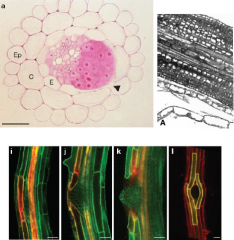
•Lateral root emergence requires separation of overlaying cells. •Auxin is promotes cell separation. Radish lateral root primordia treated with auxin. cortical cell layers have been sloughed off leaving only the pericycle surrounding the vascular cylinder. -Auxin promote cell separation. Expression of LAX3 (yellow) in cortical and epidermal cells adjacent to emerging lateral root primordia. LAX3 is an auxin influx carrier. |
|
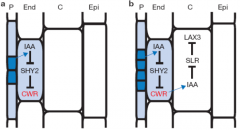
|
(a) Auxin (IAA) originating from dividing pericycle (P) cells induces cell-wallremodelling (CWR) gene expression in adjacent endodermal (End) cells. (b) Auxin derived from the developing lateral root primordium also induces expression of LAX3 in adjacent cortical cells (C). |
|
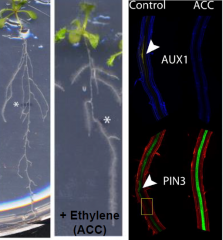
Ethylene in lateral root initation |
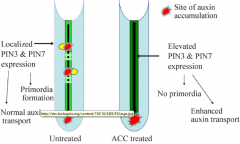
|
|
|
Leaf morphology |
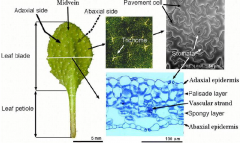
|
|
|
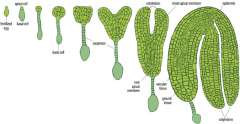
Stages of leaf development |
Stage 1: Organogenesis a few cells in L1 and L2 divide more rapidly than surrounding cells -> produce outgrowth = leaf primordium, which develops into leaf. Stage 2: Development of suborgan domains primordia acquire identity as specific parts of leaf differentiation occurs along three axes: dorsoventral (adaxial –abaxial) proximodistal (basal –apical) lateral (margin –blade –midrib) Stage 3: Cell and tissue differentiation tissues and cells differentiate as leaf grows: L1 -> epidermis with trichomes and guard cells L2 -> mesophyll cells L3 -> vascular elements |
|
|
Genetic control of leaf identity
|
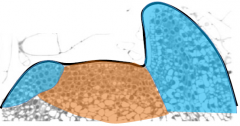
•Cells in the meristem are functionally distinct from those in the leaf primordia •They are indeterminate and serve as a stem-cell population. •Cells in the primordia are functionally distinct from those in the meristem. •They become determinate. |
|
|
KNOX-1 |
KNOX-1 genes maintain the meristem in an indeterminate state. Class I KNOX genes (KNOX-1) •KNOX means Knotted-like homeobox •Encode transcription factors •Expressed in meristem •Not expressed in leaf primordia •Help maintain indeterminate growth STM = SHOOT MERISTEMLESS KNAT1 = KNOTTED-LIKE ARABIDOPSIS THALIANA 1 KNAT2 = KNOTTED-LIKE ARABIDOPSIS THALIANA 2 KNOX genes, e.g. stm, act in part by stimulating cytokinin synthesis
|
|
|
STM |
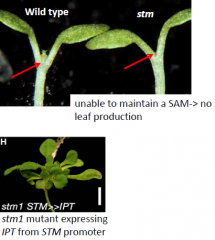
KNOX genes, e.g. stm, act in part by stimulating cytokinin synthesis. -STM is a transcription factor that induces expression of an IPTgene. -stmmutant can be rescued by CK application or by expression of the cytokinin-biosynthesis IPTgene at the SAM. |
|
|
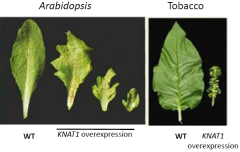
Overexpression of KNOX-1genes increases leaf complexity and indeterminacy |
|
|
|
ARP genes |

Primordium-specific genes promote differentiation •“ARP”is derived from three genes, ASYMMETRIC LEAF1 (AS1),ROUGH SHEATH2 (RS2), and PHANTASTICA •ARPgenes encode MYB transcription factors •Expressed in cells of leaf primordiaand cotyledons •Promote determinate growth and differentiation Loss of ARPgene function resembles KNOX-1overexpression.Loss of ARPgene function allows KNOX-1genes to remain on in the primordia, leading to an increased indeterminacy. Lack of AS1 function results in overexpression of KNOX-1genes. |
|
|
The activities of ARP and KNOX-1genes are mutually antagonistic |
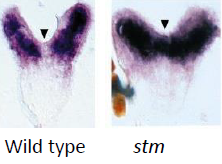
The two classes of transcription factors are mutually repressive, and help establish a separate identity for the emerging leaf primordium. In the stm mutant, AS1is expressed in the meristem (arrow).
|
|
|
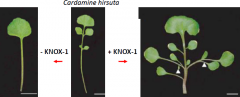
Expression of KNOX-1 transcription factors correlates with leaf complexity. |
|
|
|
In plants with compoundleaves, KNOX-1expression turns back on in primordia |
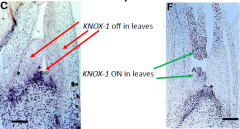
•KNOX-1genes are repressed at the site of leaf primordium initiation. •Compound leaves (e.g. tomato) KNOX-1genes turn on again in leaf primordia. •Simple leaves (e.g. Arabidopsis) KNOX-1genes stay off in the leaf primordia. |
|
|
Control of leaf complexity |

Cytokinincontributes to compound leaf development. The effects of KNOX-1 expression can be replicated by increased accumulation of cytokinin. |
|
|
Establishing leaf polarity |
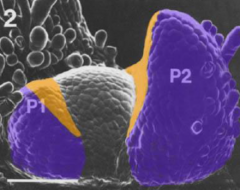
•Leaf primoridiahave inherent polarity. •The side that is closer to the meristem is adaxial. •The side that is farther away from the meristem is abaxial. |
|
|
Incipient primordia |
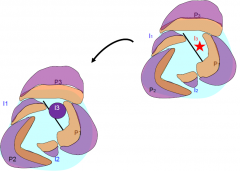
Incipient primordia is surgically isolated from the rest of the meristem by a small incision. The isolated primordium lost polarity (it became entirely abaxialized) and became radially-symmetrical. |
|
|
Laser ablation |
Laser ablation of only the epidermal cell L1 layer is sufficient for the primordium to lose its adaxial polarity. •A mobile signal from the meristem moves through the epidermis into the incipient primordium. •The signal conveys the adaxialpositional information. •Referred to as the “Sussex signal”. The nature of the signal is not known. |
|
|
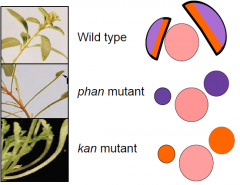
Phantastica mutant |
•The phantastica mutant has radially symmetrical leaves. •Mutant phan leaves are abaxialized, indicating that PHAN is necessary for adaxialcell fate. •PHANencodes a transcription factor. •Three redundant genes, PHABULOSA(PHB),REVOLUTA (REV) andPHAVOLUTA(PHV), function similarly to PHANTASTICA (PHAN). |
|
|
Kanadi mutants |

Loss of function kanadi mutants have radial, adaxialized leaves. •A triple mutant kanadi1,2and 3 has radial, adaxialized leaves, showing that KANADI genes promote abaxial cell fate. •This is the opposite function as PHAN. •KANADI genes also encode transcription factors. |
|
|
Loss of adaxial or abaxial fate causes radial leaves |
|
|
|
Domain-specific transcription factor expression controls polarity |
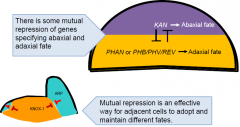
|
|
|
microRNA (miRNA) |
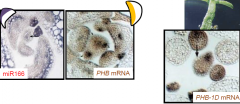
PHBexpression is regulated by a miRNA. In wild-type plants, miR166 binds to the PHB mRNA and degrades it on the abaxial side of the leaf primordium. Therefore PHB mRNA is only found on the adaxial side. In phb-1dplants, base changes in the PHB mRNA prevent miR166 from binding to it, allowing it to accumulate throughout the leaf primordium producing a radial, adaxialized leaf.
|
|
|
Meristem-derived signal |
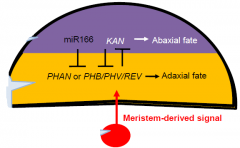
In some cases domain-specific expression is maintained by miRNAs. Different transcription factors specify adaxial and abaxial domains. The inner surface of a leaf is specified by a signal derived from the meristem. |
|
|
Control of leaf size and shape |
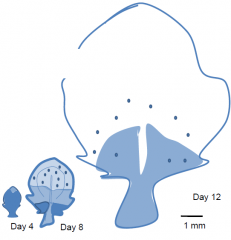
Size is determined by growth, shape is determined by differential growth. Leaf blade growth in dicots, e.g. tobacco. Leaf expansion stops first at the tip, and later at the base. The shape of the leaf is caused by more growth at the base than at the tip. Patterns of cell division correlate with blade expansion. Cell division arrests first at the tip and later at the base. Cell division is determined by transcription of a cyclin gene, assayed using a reporter construct. Darker blue indicates higher levels of gene expression. |
|

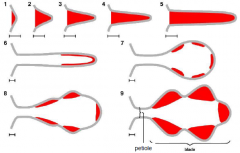
Leaf margin in dicots |
1. Smooth(entire). 2.Serrate. 3.Double serrate. 4.Saw-shaped. 5.Toothed. 6.Crenate. 7.Lobed. 8.Parted. 9. Pinnately (like a feather) incised. 10.Palmately (like a hand) incised. 11.Palmately (like a hand) lobbed. |
|
|
Cell divisions and expansion contribute to the shape of the blade. Complex leaf shapes (e.g. lobbed or serrate) are formed by persistent growth in isolated regions of the developing blade. |
|
|
Vein Order Designation |
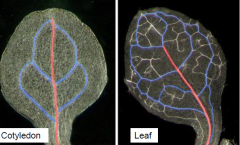
Primary vein or mid vein are in red and the secondary veins are blue. Higher order tertiary and quaternary veins have not been colored. |
|
|
Control of venation or vascular patterning |
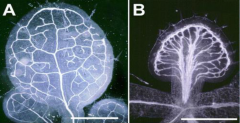
Auxin is required for proper vein patterning. Inhibition of auxin transport disrupt patterning. A: Leaf from a plant grown in normal growth medium. B: Leaf from a plant grown in the presence of a polar auxin transport inhibitor. |
|
|
After a leaf primordium is initiated, auxin moves basipetally and specifies the site of the midvein |
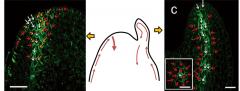
Picture on the left: Auxin redistributing in a very young primordium –arrows indicate direction of auxin transport Picture on the left: In an older primordium the future midveinis clearly demarcated. (Inset shows “top down” view) |
|
|
Second-order veins are formed by further auxin redistribution |
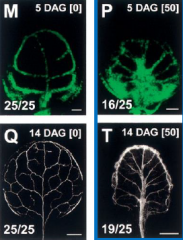
Pattern of PIN1 expression within the leaf predicts the pattern of vascular differentiation. Auxin transport inhibitors disrupt the pattern and connectivity of the vasculature in Arabidopsis leaves. |
|
|
The leaf epidermis comprises pavement cells, trichomes and guard cells |
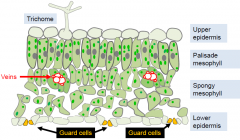
|
|
|
Guard cell structure |
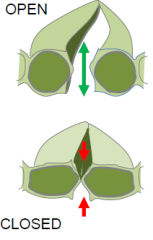
Guard cells regulate leaf pores through which water vapor and gasses move.
|
|
|
Stomatal patterning in Arabidposis |
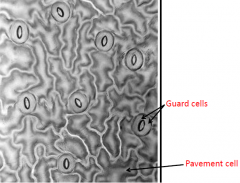
Distribution of stomata is not random; they are evenly distributed. Each stomata is typically surrounded by 3-4 pavement cells. |
|
|
Stomatal patterning in grasses |

Grass leaves have parallel veins (indicated by black arrows) separated by mesophyll cells. Guard cells form in certain cell files (indicated by red arrows) between the veins. |
|
|
“One cell spacing rule” |
Although the overall pattern of stomata on the leaf surface is variable among species, Stomata are almost universally patterned according to a one-cell spacing rule, such that at least one intervening epidermal cell separates nearby stomata from one another. |
|
|
Guard cell (GC) development in grasses |
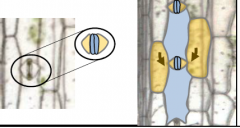
Guard cells (light blue) are supported by subsidiary cells (yellow) which form from adjacent cell files.
|
|
|
Asymmetric and symmetrical cell division |
Asymmetric division in GC file produces Guard Mother Cell (GMC). GMC induces division in adjoining cells. GMC divides to produce guard cells. |
|
|
Guard cell (GC) development in Arabidopsis |
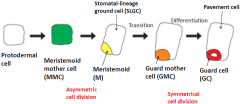
Both the patterned distribution of stomatal complexes and the differentiation of the guard cells themselves are associated with asymmetric, oriented divisions. |
|
|
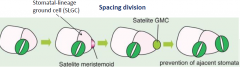
Cell division in the stomatal lineage |
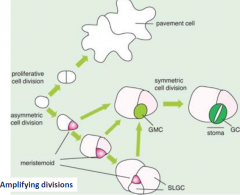
|
|
|
Meritsemoid cell |
Mesritemoid cell (P) behave as stem cell for three or four rounds of division, after which they become a GMC (A). Meristemoid cell undergo two or three rounds of division in alternating orientations creating a spiral arrangement of 3 cells with an internalized meristemoid cell. |
|
|
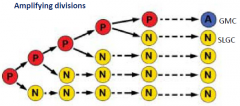
Cell division in the stomatal lineage |
|
|
|
SLGC cells |
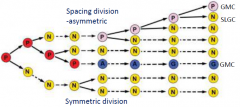
Mesritemoid cell (P) divide asymmetrically, with the smaller cell retaining P cell identity. Stomatal-lineage ground cell (SLGC) (N) cells may also divide, but such divisions are usually symmetric. SLGC (N) cells may divide asymmetrically, these cells have reacquired mesritemoid cell P identity. |
|
|
basl |
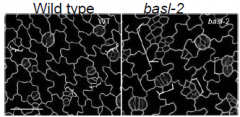
Asymmetric distribution of BASL precedes asymmetric cell division. Loss of baslfunction leads to more symmetric cell divisions. How BASL affect division polarity is not yet known. |
|
|
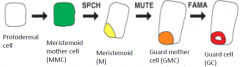
Three genes (basic-helix-loop-helix (bHLH) transcription factors) direct three sequential steps of cell-fate transition during stomatal development |
|
|
|
SPCH = SPEECHLESS |
SPCH = SPEECHLESS is required for initial asymmetric division. SPCHis expressed transiently in epidermal cells, which undergo divisions to produce meristemoids. spch mutations block stomatal lineage initiation. |
|
|
MUTE |

MUTE acts to terminate meristemoid asymmetric divisions and ensure transition to GMC. MUTE mutations results inwardly spiraling meristemoid cell divisions with aborted meristemoids in the center. Overexpression of MUTE produces an epidermis made up of mainly guard cells. |
|
|
FAMA |
FAMA = many “false mouths” of the Roman goddess of Rumor. FAMAis expressed in guard cells. FAMAcontrols the switch from the GMC to GC identity. Halt division and promote differnetiation. FAMAmutations result in reiterative symmetric divisions of GMCs, forming abnormal rows of cells (“fake mouths”) but no mature guard cells. |
|
|
Stomatal patterning is determined by inhibition of differentiation |
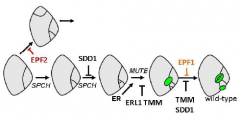
A proposed genetic pathway for stomatal patterning and development showing positive and negative regulators |
|
|
TOO MANY MOUTHS (TMM) |

TOO MANY MOUTHS (TMM) repress asymmetric divisions in cells that are next to guard cells. Required to enforce the one cell spacing rule. TMM is a leucine-rich repeat (LRR) receptor-like protein. Clusters result from disruption of the number, plane and polarity of asymmetric divisions. TMM prevents Stomatal-lineage ground cells (N) from dividing to produce a satellite meristemoid (S) adjacent to previously formed meristemoids (M). |
|
|
Mutation in ERECTA-family and YODA |
Mutation in ERECTA-family(ERf; LRR receptor-like kinases) and YODA(MAP protein kinase) genes result in elevated numbers of stomata, many of which are clustered. Required to enforce the one cell spacing rule. Required to maintain both the number and orientation of asymmetric cell divisions. |
|
|
EPIDERMAL PATTERNING FACTOR 1 and EPF2 |
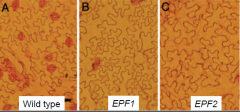
Loss-of-function mutations in two related genes encoding putative ligands,EPIDERMAL PATTERNING FACTOR 1andEPF2also confer defects in stomatal patterning. EPF1 and EPF2 regulates the orientation and frequency of division in neighboring cells (i.e. inhibits the formation of adjacent satellite meristemoid). |
|
|
Guard cell development is sensitive to exogenous factors |
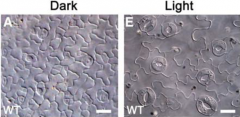
Light affects the activity of SPCH and other regulators of GC differentiation. Stomatal density is also determined by environmental CO2 levels. Transferring a plant to a higher-CO2 environment causes the newly initiating leaves to produce fewer stomata. |
|
|
Generating stomatal complex diversity |
No spiral arrangemt suggests a lack of amplifying divisions of a meristemoid. Surrounded by a spiral arrangement of cells due to a large number of amplifying divisions. Stomata arise in groups, can be explained by early symmetrical division of the MMC. |
|
|
Trichomes |
Trichomes are epidermal hairs. Some trichomesprotect plants by deterring insects and herbivores. Some trichomes reflect light to reduce UV and heat absorption. Seed trichomes such as cotton fibers aid seed dispersal. Trichomes can be unicellular or multicellular, branched or unbranched, glandular or non-glandular. |
|
|
Glandular trichomes |
Glandular trichomes produce chemicals that can have antimicrobial, insecticidal or medicinal properties. Modifying gene expression in trichomes can increase the production of chemical compounds. The plant in the center makes more of an insecticidal compound (cembratriene-ol(CBT-ol)) and is resistant to aphids. |
|
|
Arabidopsis trichomes |
•Found on upper or adaxialside of leaves, and on stems but not on hypocotyl and cotyledons. Appear as soon as the leaf primordia begins to develop. •Trichomeson leaves are branched, stem trichomesare unbranched. •Spacing between trichomesis regulated (not usually found adjacent to one another). |
|
|
Trichome morphogenesis |
Stage 1: radial expansion of the trichome precursor cell in the plane of the leaf. Stage 2: stalk emergence and expansion. Stage 3: formation of branch structures (primary branching). Stage 4: branching (secondary branching), expansion of the stalk and branches which have blunt tips. Stage 5: continued expansion of the stalk and branches, which develop pointed tips. Stage 6: mature trichome with papillate surface; cell walls thicken, accessory cells differentiate around the base. As trichomes differentiate, they endoreduplicate, causing their ploidy(DNA content) to increase from 2n to 32n. The cell cycle of endoreduplicatingcells skips mitosis. Number, spacing and shape of trichomesis tightly regulated. |
|
|
Trichome spacing |
Some mutants under produce trichomes (like gl1). Some mutants overproduce trichomes (like cpc try). |
|
|
TRIPTYCHON (TRY) |
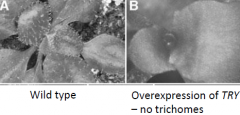
TRIPTYCHON (TRY) mutation cause clusters of trichomes. TRYis an inhibitor of trichome development. |
|
|
Trichome shape |
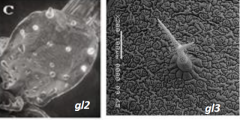
GLABRA(GL) and TRANSPARENT TESTA GLABRA1 (TTG1 ) mutants GL1and TTG1mutation: complete absence of trichomes GL2 mutation: small trichomes with little to no branching GL3mutation: reduced number of trichomes that are smaller and have fewer branches.
|
|
|
Trichome mutants |
|
|
|
GL1, GL3 and TTG complex regulate trichomecell fate specification |
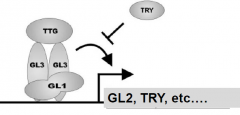
•GL1encodes a MYB transcription factor. •GL3encodes helix-loop-helix protein –transcriptional regulator. •TTG1 encodes a WD40 domainprotein-transcriptional co-regulator. •Complex activates the expression of GL2, a homeoboxtranscription factor and TRY, a MYB-like protein. TRY inhibits the activity of the complex. GL2 turn on trichomespecific genes. |
|
|
Trichome patterning |
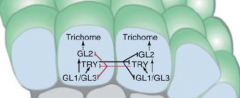
•All epidermal cells have equivalent developmental potential. •Each use TRY to inhibit its neighbor –lateral inhibition. |
|
|
Expression of TTG1, GL1 and GL3 increases some cells. Result…
|
-Increase in TRY activity. -Inhibition of differentiation in adjacent cells. -trichome development. |
|
|
In Arabidopsis, trichome spacing is controlled by an inhibitory signal |
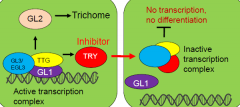
A transcription complex activates expression of the trichome inducer GLABRA2 (GL2)..... and a mobile inhibitor moves into adjacent cells to inhibit GL2 expression –lateral inhibition. GL2expressing shoot epidermal cells adopt a trichomecell fate. |
|
|
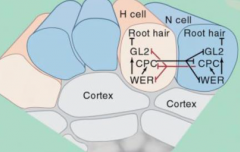
Root hair |
Root hair is an extension of an epidermal cells. Trichoblasts root epidermal cells that produce a root hair. |
|
|
Root hair patterning |
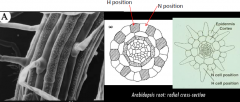
•Determined by position relative to neighboring cortical cell. •Trichoblasts: root hair cell, H position, forms at cortical cell junction. •Atrichoblasts: non-root hair cell, N position, all the cells between. |
|
|
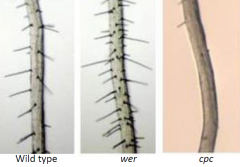
•Three mutants, werewolf (wer), ttg, and gl2, produce root hairs on essentially every root epidermal cell. •CAPRICE (CPC) mutation reduce the number of root hairs. |
|
|
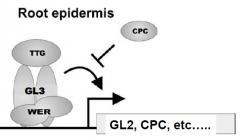
WER, GL3 and TTG complex regulate root hair cell fate specification. •WERencodes a MYB transcription factor, similar to GL1. •CPCencodes a protein similar to TRY. •Complex activates the expression of GL2 andCPC. CPC inhbit the activity of the complex. GL2 turns OFF root hair genes. GL3 |
|
|
•All epidermal cells have equivalent developmental potential. •Each use CPC to inhibit their neighbors –lateral inhibition. •Expression of WER increases in N cells. •Result…. -Increased CPC activity (N cells). -CPC inhibit of GL2 expression in adjacent cell (H cell). -Adjacent cell forms root hair (H cell). |
|
|
In Arabidopsis, root hair spacing is controlled by an inhibitory signal |
A transcription complex activates expression of the root hair inhibitorGLABRA2 (GL2)..... and a mobile inhibitor moves into adjacent cells to inhibit GL2 expression –lateral inhibition. GL2expressing root epidermal cells (N position) adopt a non-root-hair cell fate. |
|
|
How does the hair cell form only at the cortical cell junction??? |
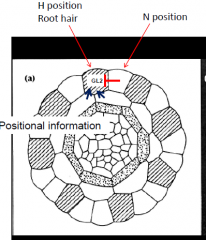
Inhibition of GL2 is biased towards the epidermal cell located at the cortical cell junction. Inhibit GL2 expression –root hair develops. Look for a mutant where root hairs are randomly formed = SCRAMBLED |
|
|
Scrambled (SCM) mutant |
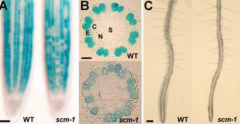
N position cells produce root hairs. Blue = GL2 expression , expressed in N position -NO root hair. •SCRAMBLED (SCM) is a epidermal cell-localized receptor. •High levels of an unknown signal produced by the root cortical cells activate SCM. •SCM negatively regulates WERexpression. •SCM activity in H cell position bias the outcome of lateral inhibition. |
|
|
Spores, gametophytes, gametes |
Spores •Megaspore (female) •Microspore (male)
Gametophytes •Embryo sac (female) •Pollen grain (male)
Gametes •Egg cell (female) •Sperm cell (male) |
|
|
Pollen Grain Structure: Germinated Pollen Grain |
Pollen grain (gametophyte) |
|
|
Pollen Grain Structure: Pollen grain (gametophyte) |
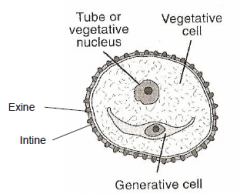
|
|
|
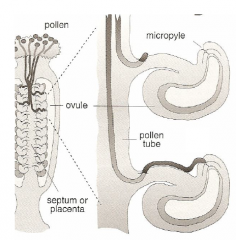
Ovule Structure - Arabidopsis |
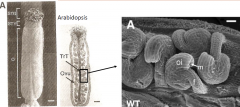
Female reproductive organ (pistil) contains an ovary with two fused carpels (gynoecium). Each carpel contain ovules.
Trt –transmiting tract Ovu –ovule Stg-stigma Sty –style
f -funiculus oi-outer integument m -micropyle O –ovary |
|
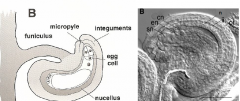
|
cn, central cell nuclei; en, egg cell nucleus; ii, inner integument; n, nucellus; oi, outer integument; sn, synergid nucleus.
|
|
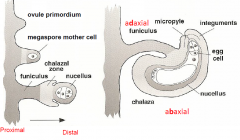
Ovule Development |
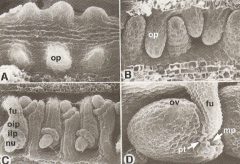
op –ovule primordium ov–ovule fu-funiculus oip-outer integument primordium iip –inner integument primordium nu -nucellus mp-micropyle Pt –pollen tube |
|
|
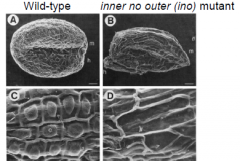
ino mutant |
|
|
|
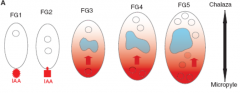
Auxin (IAA) dependent cell specification |
|
|
|
Pollination and fertilization |

1. Pollen lands on the stigma, where it hydrates, and germinates. 2. Growing pollen tube penetrates the cuticle and cell wall of papillarcell and grows down the transmitting tract (septum). 3. In the ovary, tube emerges from the transmitting tract , grows along funiculusand enters ovule, where it fertilizes the egg and central cells. |
|
|
Pollination |
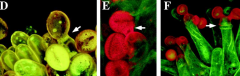
1.Pollen adhesion 2.Pollen coat “foot” formation and pollen hydration 3.Pollen tube emergence (germination) 4.Pollen tube invasion of the papillae cell wall and extension towards the style. |
|
|
Compatible vs. Incompatible Pollination |
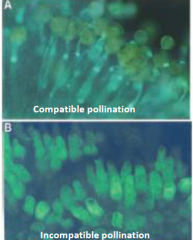
• A genetic “checkpoint” for the rejection of self pollen. •Pollen germination only occurs on a compatible stigma. • Incompatibility promotes cross-pollination/outcrossing. |
|
|
Types of Self-incompatibility: Gametophytic incompatibility |
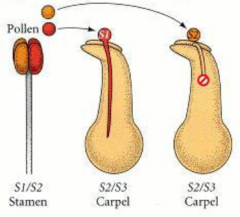
|
|
|
Types of Self-incompatibility: Sporophytic incompatibility |
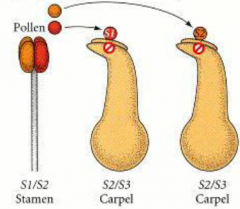
|
|
|
Protein Ubiquitination |
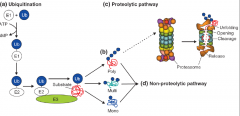
|
|
|
Pollen Tube Growth |
|
|
|
Double Fertilization |
Results from the discharge of two sperm cells from the pollen tube into the embryo sac. 1) sperm cell + egg cell ->diploid zygote 2) sperm cell + central cell ->triploid endosperm |
|
|
Double Fertilization Steps |
Step 1 is the pollen tube reception and discharge phase. Sperm cells are delivered from the pollen tube into the female gametophyte. The receptive synergidcell breakdowns (cell death) just after the start of pollen tube discharge. Step 2 is the immobility phase. After delivery, sperm cells remain at the boundary region between the egg cell and the central. Step 3 is the double-fertilization phase. Sperm cells fuse with each target cell. |
|
|
Pollen Tube Reception and Discharge |
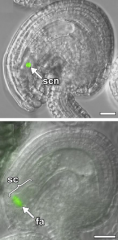
The synergidcell (SC) wall forms a highly thickened structure called the filiform apparatus (FA) at the micropylarend, consisting of finger-like projections that extended into the synergidcell cytoplasm. Pollen tube (PT) grows through the filiform apparatus (FA), stops growth, and ruptures to release the two sperm cells. The synergidcell (SY) that receives the PT undergoes cell death (DSY). Cell death facilitates the fertilization process. |
|
|
Arabidopsis lyrata x Cardamine flexuosa |
In crosses with Arabidopsis lyrata, (closely related) and Cardamineflexuosa, (distantly related) pollen tubes that entered a wild-type A. thalianaembryo sac continued to grow inside the female gametophyte -reception failed. |
|
|
ANXUR (ANX)1and ANXUR2 |
ANXUR (ANX)1and ANXUR2 •Male components controlling pollen tube reception •Expressed within the growing pollen tube •Mutants are male sterile •Pollen tube discharges prematurely |
|
|
Gamete Fusion |
• Generative Cell Specific1 (GCS1/HAP2) • Wid type ovules following targeting by wild type (D,E) or hap2 (F,G) pollen tubes. • Two sperms are released into the degenerating synergid but fertilization does not occur. |
|
|
Pollen Tube Guidance |
•Pollen-tube growth involves attraction and guidance mechanisms acting along the apical-basal axis andalong lateral axes toward ovules. •Transmitting tissue provides and nutrient support and chemical gradients or guidance cues for pollen tube growth. •Ovule also provides guidance cues. |
|
|
Pollen Tube Guidance: nttmutant |
•A properly developed tract is required for tube growth. •no transmitting tract (ntt)abnormal tract. •Pollen tubes grow slowly and exit the tract early (decrease in apical-basal growth) |
|
|
Pollen Tube Attraction |
Stigma an style confer competence. Pollen tube that did not go through stigma cannot target ovule. That is pollen tubes acquire the ability from pistil tissue to perceive ovule guidance signals. Pollen-tube guidance to the ovule is governed by the targeted female gametophyte. In vitro assay: GFP-tagged pollen tubes make a committed turn (arrows) before entering a ovule and lysing (arrow heads). Attraction of the pollen tube to the naked embryo sac. Embryo sac emits a diffusible signal. |
|
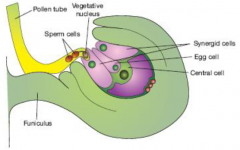
|
•Embryo sac contains 7 cell types. •Which cell type provides the guidance cue? •Most likely candidates are the egg cell and synergid cells located next to the micropyle. |
|

|
Science 2001. 293(5534): 1480 - 1483 • Laser ablation of each gametophytic cell in the embryo sac. • Egg/central cell ablated - attraction not affected. • Two synergid cells ablated - no pollen tube attraction. The synergid cells attracts to the growing pollen tube. |
|
|
LURE |
Pollen tubes grown through a cut style (competent) is attracted to LURE coated bead.
Pollen tubes germinated in vitro(noncompetent pollen tubes) is not attracted to LURE coated bead.
LUREs (LURE1 and LURE2) are expressed specifically in the synergid cell. •secreted toward the micropylar end of the embryo sac. •localized in the filiform apparatus (structure at the base the synergid cells. Cell |
|
|
Block to polytubey |
Only one pollen tube enters each micropyle. |
|
|
Fertilization recovery mechanism |
A “block to polytubey” is triggered by gamete fusion. If defective sperms are deposited, additional pollen tubes are attracted. The second synergidcell accepts a pollen tube. Fertilization is recovered. |
|
|
Flower architecture |
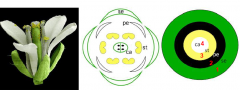
•Arabidopsis flowers are arranged in whorls -Four concentric whorl of organs -Regular pattern of number and position Flowers always have radial or bilateral symmetry |
|
|
Modifications when making a flower |

Shoot -> Flower Modifications: •No leaves below flowers •Change in phyllotaxy (whorl instead of spiral) •Very short internodes •No branching •Change in organ identity from leaves to floral organs •Determinate growth instead of indeterminate growth (organ production stops after carpels are made) Overall, all a plant does is convert a shoot into a flower, have whorls instead of shoots, and very short internodes. |
|
|
Four class of genes are involved in flower development |
Flowering time genes: regulate the transition from vegetative (when it is just producing leaves) to reproductive growth (when it produces flowers). Meristem identity genes: specify and maintain floral meristem identity. Flower organ identity genes: determine the fate of organ primordia. Now having different organs instead of just primordia Late-acting genes: control ovule development |
|
|
Regulatory cascade of flower development |
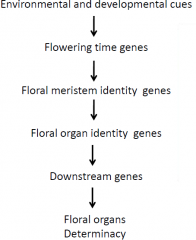
It's a cascade, one step turns on the first and those turn on the next. |
|
|
Control of flowering time |
•Multiple signals regulate flowering in Arabidopsis. •Ultimate result of signals: turn on floral homeotic genes. SOC1 has to be expressed first, with conditions such as leaf number, temperature, photoperiod, etc. The outcome is SOC1 gets turned on, and that turns on LFY and AP1, and that causes flower homeotic genes. Once you turn on LFY and AP1, |
|
|
Once you turn on LFY and AP1 |
As plants initiate reproductive development, the vegetative meristem is transformed into an intermediate inflorescence meristem that produces floral meristems on its flanks. |
|
|
Floral meristem |
•Floral meristem are determinate, produce a defined number of organ primordia. •Floral organs are: -initiated sequentially by the floral meristem -produced as successive whorls (concentric circles) Once you've initiated floral meristem, you star determining what is going to be sepal, petal, etc. |
|
|
Two distinct classes of genes for floral meristem identity |
1.Promotes floral meristem identity. Two key regulators LEAFY(LFY) and APETALA1 (AP1). Ensures that primordiainitiated along the periphery of an inflorescence meristem adopt a flower fate. 2.Maintains the identity of inflorescence shoot meristems. Includes TERMINAL FLOWER. |
|
|
leafy (lfy) and apelata1 (ap1) single mutants |
•Partial conversion of flowers into shoot •lfymutant: spiral phyllotaxy in flowers (not perfect whorl) and floral organs look like leaves, instead of having petals and primordium. •ap1mutant: branches are formed in flower and leaves instead of sepals, no petals.
|
|
|
Environmental factors such as age of plant, etc turn on LFY and AP1 |
which turn on organ primordoa |
|
|
apetala 1 leafy doublemutant |
•“Flowers” lack most flower characteristics (still have short internodes). •Lots of leaves, no floral organs, spiral phyllotaxy (no whorls). •Indeterminate growth. |
|
|
agamous mutants |
•agamousmutants show indeterminacy. •In the flower LEAFY (LFY) and WUSCHEL (WUS)activate AGAMOUS (AG). •AGAMOUS then turns off WUSCHEL, causing cessation of floral meristem growth. So if you lose LFY, you lose determinancy, so the flower keeps growing. |
|
|
What genes are involved in specification of particular organs? |
•Find plants that have organs incorrectly specified for their position (e.g. petals converted to sepals). •These mutations represent a defect in the ability of cells to correctly asses their position, so they differentiate incorrectly. |
|
|
Mutations in the floral identity genes: 5 genes identifies |
Each mutant effects two whorls. The 5 genes are apetala1(ap1), apetala2(ap2), pistillata (pi), apetala 3 (ap3), agamous (ag) |
|
|
The ABC model of flower development |
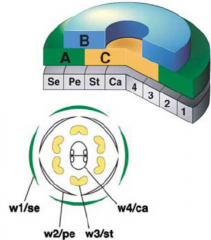
•3 classes of genes A, B and C. •Each class act on two whorls (w). Function combinatoriallyto specify organs. •A alone = sepals (w1) •A+B = petals (w2) •B+C = stamens (w3) •C alone = carpels(w4) |
|
|
3 classes of genes A, B and C |
If you lose agamous the expression of A expands into whorls 1 and 2. •A function: AP2, AP1 •B function: AP3, PI •C function: AG •A and C are mutually exclusive. •C function genes are required for the production of stamens and carpel . •agamous (ag) = no gametes ag mutants: AP1/2 is expressed in all four whorls. stamen replaced by petals, carpels replaced by sepals . Indeterminacy –more whorls are formed. |
|
|
A function genes |
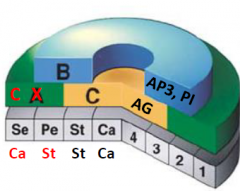
•A function genes are required for the production of sepals and petals. •apetala2 (ap2), apetala (ap1) •ap2 mutant: AG is expressed in all four whorls. Sepals are converted to carpels and petals are converted to stamens. |
|
|
B function genes |
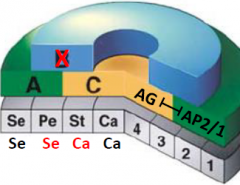
•B function genes are required for the production of petals and stamens. •apetala3 (ap3), pistillata (pi) mutants: both mutations give the same phenotype. Petals replaced by sepals, stamens replaced by carpels |
|
|
C function genes |
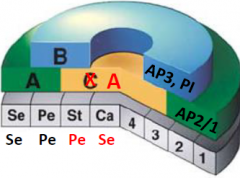
•C function genes are required for the production of stamens and carpel . •agamous (ag) = no gametes ag mutants: AP1/2 is expressed in all four whorls. stamen replaced by petals, carpels replaced by sepals . Indeterminacy –more whorls are formed |
|
|
MADS box proteins |
•MADS box proteins are required for floral development but they are not sufficient. Modifying the ABC model •Plants lacking all three classes of ABC gene activities formed flowers that had only leaf-like organs (default identity). •Overexpression of ABC genes, alone or in combination, failed to convert leaves into floral organs. |
|
|
SEPALLATA (SEP) genes |
•A fourth class, the SEPALLATA (SEP) genes, is also required organ identity. •SEPALLATA 1-4 (SEP1-4) genes are expressed in all four whorls. •SEPgenes combination loss of function -produces leaf like organs. •Overexpression of SEPgenes in combination with ABC genes (AP1, AP3, PI ) lead to transformation of vegetative leaves into floral organs. |
|
|
Floral homeotic genes |
•All encode MADS box transcription factors. •Activate organ-specific gene expression in a combinatorial manner. •Dimerization of MADS box proteins supports the ABCE model. |
|
|
agamous pistillata double mutant |
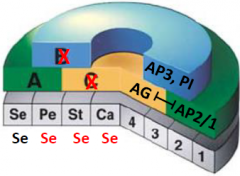
•No B and C function •A function spreads throughout the flower –all sepals •Indeterminacy due to loss of AGAMOUS |
|
|
apetala2 pistillata double mutant |
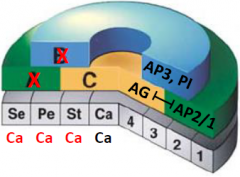
•No A and B function •C function spreads throughout the flower •All carpels |
|
|
apetala2 agamous double mutant |
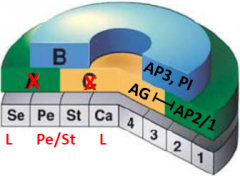
•No A and C function •Model does not predict what organs may form when only B function is present. |
|
|
Overexpression of AGAMOUS (AG) in all four whorls |
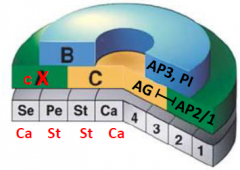
•C function in all four whorls. •Loss of A function in whorl 1 and 2. •Whorl arrangement is maintained. |

