![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
260 Cards in this Set
- Front
- Back
|
Development |
The slow process of PROGRESSIVE change that give rise to a multicellular organism.
Developmental biology studies the initiation and construction of an organism rather than their maintenance. |
|
|
Differentiation |
How cells become different from one another and from their precursors. Each cell becomes structurally and functionally different producing distinct cell types, e.g. blood, muscle or skin cells |
|
|
Morphogenesis |
The biological process that causes an organism to develop its shape. It is one of three fundamental aspects of developmental biology along with the control of cell growth and cellular differentiation. In morphogenesis cells migrate, alter size and shape, tissues fold and separate into ordered structures. For example during gastrulation, the ball of cells (blastula) is rearrange into a gastrula, cells on the outside of the embryo moves inside, end result is the formation the germ layers, the endoderm, ectoderm and mesoderm. |
|
|
Cleavage |
Cell division is tightly regulated process. Each cell divides at the right time and place. The fertilized egg (zygote) rapidly divides into a number of smaller cells forming the blastula - Cleavage. |
|
|
Pattern formation |
Overall body plan is laid down (e.g. body axes are specified). |
|
|
Phenotypic plasticity |
An organism can express one phenotype under a certain set of condition and another phenotype under other conditions. This is an example of how the environment can influence development. Phenotypic plasticity occurs frequently in plants. |
|
|
Fin-to-limb transition |
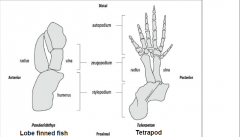
-Devonian period, 400-600 million years ago |
|
|
Some characteristics of a model organism |
1)Size allows for easy manipulation and storage 2) Easily available and inexpensive 3) Short generation time 4) Mutants are easily generated, genetically modified (genetics) 5) Genomics information is available |
|
|
Examples of animal model organisms |
• Worm, Caenorhabditis elegans |
|
|
Embryology |
The branch of biology that deals with the development of an embryo from the fertilization of the ovum to the fetus stage. |
|
|
Factors in choosing a model system |
Embryology- •Bigger is often better for these experiments Cell Biology and Microscopy- •Need to deal with protective layers (egg shell, vitelline envelope) •Ease of fixation and staining Genetics- •Need to grow for many generations or indefinitely in lab Genomics- •Low genome complexity (less “junk” DNA and smaller regulatory regions). •Low amount of gene redundancy makes forward and reverse genetics easier. |
|
|
Forward versus reverse genetics |
•Forward genetics (mutational analysis) - need to keep a large number of families in a small space |
|
|
Transgenetics |
The ability to put back new or modified genes into genome. |
|
|
Advantages and disadvantages of worms as a model organism (C. elegans) |
• Advantages |
|
|
Advantages and disadvantages of "Fruit fly: D. melanogaster" as a model organism |
•Advantages |
|
|
Advantages and disadvantages of "Zebrafish: Danio rerio" as a model organism |
• Advantages |
|
|
Advantages and disadvantages of "African clawed frog: Xenopus laevis" as a model organism |
• Advantages |
|
|
Advantages and disadvantages of "Chick: Gallus gallus" as a model organism |
• Advantages |
|
|
Advantages and disadvantages of "Mouse: Mus musculus" as a model organism |
• Advantages - Genetic system that is •Disadvantages
|
|
|
Piebaldism syndrome |
Sterility, deafness, patching skin |
|
|
Aristotle considered two basic developmental questions: |
-Do all parts of a developing organism come into existence together and simply grow larger? |
|
|
Preformation |
The organism is preformed as a complete miniature structure in the sperm or the egg and simply grows larger as it develops. |
|
|
Homunculus |
A miniature, fully formed human. Nicolas Hartsoeker (1694) |
|
|
Epigenesis (upon formation) |
New structures arose progressively through a number of different stages. Organs of the embryo form “de novo”. Aristotle was a proponent of this theory. |
|
|
Cell Theory |
-Proposed by Theodor Schwann and Matthias Schleiden (1838) 1. All living organisms are composed of one or more cells.2. The cell is the most basic unit of life. 3. All cells arise from pre-existing, living cells. (this part was added by Rudolph Virchow's (1855) |
|
|
Germ-Plasm Theory |
•August Weismann's (1880’s) theory of the germ plasm, which proposed a segregation between germline (germ cells) and somatic (body) cells during development. |
|
|
Anatomical approach to developmental biology |
Describe embryogenesis (comparative/descriptive) |
|
|
Experimental approach to developmental biology |
Manipulate the embryo by cutting, grafting, etc. |
|
|
Genetic approach to developmental biology |
IsoQlate mutations that change development. |
|
|
Quantitative approach to developmental biology |
Mathematical modelling, computational |
|
|
Ectoderm |
-(outside) outer layer of embryo -The ectoderm is the start of a tissue that covers the body surfaces. It emerges first and forms from the outermost of the germ layers. -The nervous system and the skin derive entirely from the ectoderm. The ectoderm also gives rise to the neural plate. |
|
|
Endoderm |
-The endoderm is one of the germ layers formed during animal embryogenesis. Cells migrating inward along the archenteron form the inner layer of the gastrula, which develops into the endoderm -The endoderm is the innermost layer -The endoderm ultimately gives rise to the lining of many of the internal organs (viscera)
|
|
|
Mesoderm |
-From the mesoderm arise the bones of the skeleton and the muscles. -Middle layer -The mesoderm germ layer forms in the embryos of animals more complex than cnidarians, making them triploblastic. During gastrulation, some of the cells migrating inward contribute to the mesoderm, an additional layer between the endoderm and the ectoderm. |
|
|
Triploblastic |
-Organism that has all three layers in the embryo (ectoderm, endoderm, mesoderm)
|
|
|
Diploblastic |
-Some phyla such as the cnidarians (sea anemones, hydra and jelly fishes) lack a true mesoderm and are considered diploblastic animals. |
|
|
Fate Maps |
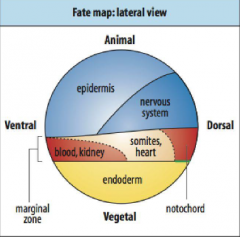
-First developed by Walter Vogt (1929) -A method to label a small number of cells with a dye that would be passed on to all of those cells' direct descendants. Follow the marked cells through development to see what they became. |
|
|
How are cells labelled in fate maps? |
-C3 cell injected with a fluorescent die, give rise to mesodermal cells on one side of the embryo. |
|
|
Early ablation experiments in experimental embryology |
Destroyed right or left halves of frog embryos. Obtained “half embryos” having a complete right or left side. Wilhelm Roux (1888) concluded that the fate of each cell is determined at cleavage. |
|
|
Mosaic Development |
Embryo constructed of individual modules capable of self-differentiation. Cells developed autonomously. |
|
|
Theory of nuclear determination |
•Theory put forward by August Weisman (1880’s) provided suppport for mosaic development. |
|
|
Was the theory of nuclear determination correct? |
•Weisman’s theory of nuclear determinants is incorrect. However the distribution of cytoplasmic determinants at cleavage do make daughter cells different from each other. |
|
|
Early isolation experiments in experimental embryology |
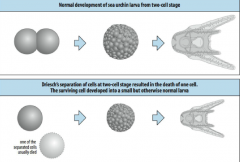
At the two cell stage, Hans Driesch (1892) killed one of the two cells when the embryo was at the two cell stage. The surviving cell developed into a small but otherwise normal larva. |
|
|
Regulative Development |
The ability of embryonic cells to change their fate so as to compensate for missing parts |
|
|
Is development mosaic or regulative? |
Virtually all embryos undergo both mosaic and regulative development at some point during their growth. |
|
|
Gene expression determines cell behavior and development |
1. Cell-cell communication 2. Cell shape changes 3. Cell movement 4. Cell proliferation 5. Cell death (apoptosis) |
|
|
Genes expression regulated at several levels |
1. Transcription |
|
|
Cell Identity |
-Differential Utilization of the Genome -Specific Pattern of Gene Expression and Genes that can be Expressed |
|
|
Maternal-effect genes |
-Genes active in the mother whose products are loaded into the egg and are required for the earliest developmental stages. |
|
|
Zygotic genes |
Genes active in the embryo, required for its own development. The embryo’s own (zygotic) genes become activated sometime during cleavage. |
|
|
Chimera |
-Organism made up of cells from two different sources. -If the mutation is present in the germ line the chimera will produce gametes that carry the mutation. Chimera is bred to produce strains of heterozygote transgenic mice. |
|
|
Reverse Genetic Approaches |
-Gene Knock-out (mice) -RNA Interference (RNAi) – introduction of double stranded RNA (dsRNA) triggers the destruction of mRNA of similar sequence. |
|
|
Ectopic Gene Expression |

-Gene is expressed where it is not normally found. -Aniridia: absence of the colored -Ectopic expression of eyeless |
|
|
Neural tube formation |
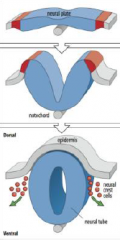
The neural plate gives rise to the neural tube and neural crest cells. |
|
|
Outer ectoderm (epidermis) - ectodermal derivatives |
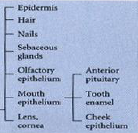
|
|
|
Neural crest - ectodermal derivatives |
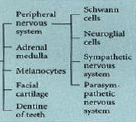
|
|
|
Neural tube - ectodermal derivatives |

|
|
|
Neural Induction |
Interaction between the dorsal lip of the blastopore (the organizer) and the neighboring ectoderm leads to the induction of the nervous system. |
|
|
Neural Induction: Default model |
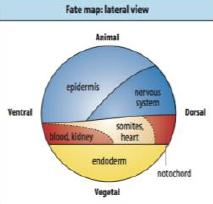
• Ectodermal cells will become neurons if they receive no signals. |
|
|
BMP4 |
Regulates the formation of teeth, limbs and bone from mesoderm. It also plays a role in fracture repair, epidermis formation, dorsal-ventral axis formation, and ovarian follical development. • Neural fates is inhibited in prospective epidermal cells by the action of bone morphogenetic proteins (BMPs).
|
|
|
Noggin |
-An organizer molecule which inhibits BMP4 Noggin is a signaling molecule that plays an important role in promoting somite patterning in the developing embryo.[3] It is released from the notochord and regulates bone morphogenic protein during development.[4] The absence of BMP4 will cause the patterning of the neural tube and somites from the neural plate in the developing embryo. It also causes formation of the head and other dorsal structures |
|
|
Effect of ectoderm, BMP, and inducers on |
Ectoderm alone --------> Neural |
|
|
How do noggin and chordin block BMP? |
Noggin and Chordin inhibit BMP by blocking there interaction with the receptor in the ectoderm. They signal from the ventral mesoderm with inducers BMP-4 and Xwnt-8. |
|
|
Frizbee |
Also a neural inducer, involves epidermal inducers such as BMP-4, and leads to epidermis. |
|
|
How do neural inducers work during gastrulation? |
Inhibitory proteins (noggin/chordin) from the organizer act on adjacent ectoderm at the beginning of gastrulation and cells derived from the organizer (notochord) influence adjacent ectoderm as gastrulation continues. |
|
|
How is the neural tube regionalized? |
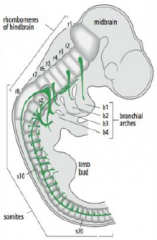
•The neural tube bulge and constrict to form the chambers of the brain (mid, hind and forebrain) and the spinal cord. |
|
|
Signaling centers of the brain |
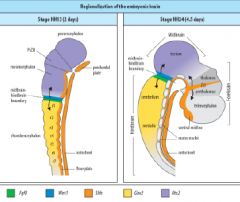
ZLI – Zona limitans intrathalamica |
|
|
Hindbrain segmentation |
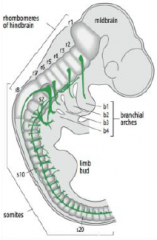
Hindbrain is segmented along the antero-posterior axis into eight rhombomeres, r1-r8. |
|
|
Rhombomeres |
In the vertebrate embryo, a rhombomere is a transiently divided segment of the developing neural tube, within the hindbrain region (a neuromere) in the area that will eventually become the rhombencephalon. Rhombomeres determine the pattern of the following maturation of the rhombencephalon into its final parts. The final parts are defined as the pons, cerebellum and medulla. |
|
|
Hox genes are members of the homeobox gene family |
• Regulate developmental processes in diverse organisms (yeast, plants, insects, mammals) |
|
|
Homeosis |
• Coined in 1894 by W. Bateson |
|
|
Homeotic transformation |
Example, changing antennas into legs (Antennapedia (Antp)) in a fly or adding an extra pair of wings (Bithorax (bx)).
|
|
|
How Do Homeotic Genes Function? |
•Act as genetic switches that control the choice between different developmental pathways. Segments where Antp is required for leg formation it disengages antenna genes and a leg is formed. |
|
|
Homeobox genes exhibit two patterns of localization: |
1.Some are scattered throughout the genome. |
|
|
Hox complexes |
Arose by repeated duplication and mutation of an ancestral homeobox gene. This formed an ancestral HOX complex. In some organisms, including most vertebrates, the HOX complex has been duplicated four times. |
|
|
Paralogy group |
Hox genes are organized into paralogy and orthology groups. Are simply the natural clusters of Hox genes on the chromosome. Vertebrates have 4, flies have 1. They are named a, b, c, and d. |
|
|
Orthology group |
Hox genes are organized into paralogy and orthology groups. Sequence comparisons of each gene shows that certain genes are closely related. These are the genes in most vertical columns (for example, Hox a9, b9, c9 and d9). Orthologous Hox genes from different species can replace one another in function (Hox d4 substitutes for Dfd in Drosophila). Genes of any orthology group have corresponding positions within each complex (if clusters are aligned in rows, the orthology groups form columns. Genes are numbered consecutively from 1-13. |
|
|
How do hox gene expression along the antero-posterior axis |
The physical order of Hox genes within the complex is related to their order of expression along the antero-posterior axis of the embryo!! (colinearity). Genes at the 3’ end are expressed in the anterior and genes at the 5’ end are expressed progressively further posteriorly. |
|
|
Hoxb cluster |
Genes are expressed in the neural rube and mesoderm. each gene is first expressed at a sharply defined point and expression continues posteriorly and gradually tapers off. |
|
|
Homeotic transformations |
-Conversion of one body part to another -Result from Hox gene loss or over expression. |
|
|
Posterior prevalence |
Mutation affects the anterior extent of gene expression. Hox gene loss leads to cells assuming a "more anterior value" i.e. Hoxc8 mutant mice have extra ribs. |
|
|
Expression in hox genes for r1 and r2 |
No Hox genes are expressed in r1. Expression of Hoxb1 in r2 results in motor axons going to b2 instead of b1 -homeotic transformation. |
|
|
Retinoic Acid (RA) |
• Retinoic acid is a derivative of vitamin A |
|
|
Retinoic acid in mouse embryo |
Retinoic acid activated gene expression: Result – gene expression is activated throughout forebrain.
Normal mouse brain: Abnormal brain of a mouse whose mother ingested this same amount of retinoic acid. |
|
|
Retinoic acid acts as both a teratogen and an endogenous signaling molecule |
•Excessive amounts of vitamin A or a vitamin deficiency results in severe brain abnormalities |
|
|
Dorso-ventral Organization of the Neural Tube |
•Neural tube that develops into spinal cord is pattern along the |
|
|
Sonic hedgehog |
A ventral signal in dorsal-ventral patterning. It occurs in the spine and patterns the ventral half of the tube. Notochord emits sonic hedgehog that induces floor plate and motor neurons. BMP is the dorsal signal.
|
|
|
Lateral inhibition |
In neurobiology, lateral inhibition is the capacity of an excited neuron to reduce the activity of its neighbors. Lateral inhibition disables the spreading of action potentials from excited neurons to neighboring neurons in the lateral direction. |
|
|
Notch and delta signaling |
• Specification of vertebrate neuronal precursor involves lateral inhibition. • Cells can adopt a neural or epidermal fate. |
|
|
Examples of axial planes |
Ventral-pertaining to the belly. The opposite of dorsal. |
|
|
Developmental Processes |
Cell Division: producing a multicellular embryo involves an initial cleavage stage followed by regular mitotic divisions. |
|
|
Sea Urchin Cleavage |
Cleavage: Egg divides assymetrically producing two cell types; macromeres and micromeres. Blastula consists of cilliated epithelial monolayer surrounding a fluid-filled cavity called blastocoel. |
|
|
Sea Urchin Gastrulation |
Gastrulation: compromises a series of cell rearrangements in which the primary germ layers move into their positions and the basic body plan is established, forms a gastrula. Events in gastrulation |
|
|
Characteristics of frog egg |
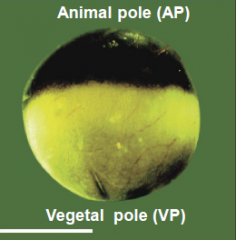
Egg is divided into a dark pigmented hemisphere (the animal pole) and a lightly or unpigmented hemisphere (the vegetal pole). |
|
|
Early frog development: cleavage |
Cleavage: Egg is divided into smaller and smaller cells called blastomeres (macromeres and micromeres). Fluid filled blastocoel (Bc) develops in the animal pole (AP) of the blastula. |
|
|
Early frog development: blastula |
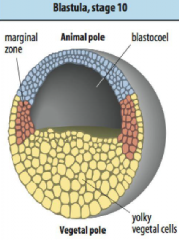
• Blastula produced after 12 divisions. |
|
|
Cells that will give rise to … |
….ectoderm is at the animal pole. |
|
|
Early frog development: gastrulation |
Gastrulation: Extensive rearrangement of the embryo so as to place the germ layers in the correct position. Internalization of the mesoderm and endoderm and spreading of the ectoderm. Basic phases of frog gastrulation. |
|
|
By the end of gastrulation... |
1) 3 germ layers are in position. |
|
|
Early frog development: neurulation |
Neurulation: formation of the neural tube, the precursor for the central nervous system. Neural crest cells are also created. Embryo now called a neurula. Events in neurulation |
|
|
Formation of a neural tube |
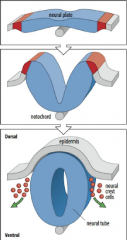
1) Formation of a neural plate in the ectoderm located above notochord. 2) The edge of the neural plate forms neural folds which rise towards midline. |
|
|
Early chick development: cleavage |
Cleavage: occurs in small patch of cytoplasm atop the yolk. Produces a blastoderm. |
|
|
Hypoblast, epiblast, and koller's sickle |
Hypoblast – develops over the yolk. Source of extra-embryonic tissues. |
|
|
Early chick development: gastrulation |
Gastrulation: Allocation of germ layers. Begins with the formation of the primitive streak, a furrow in the epiblast. 1) Hypoblast is replaced by endoblast. Primitive streak begins at the posterior marginal zone specifically at the Koller’s sickle. |
|
|
Visualization of gastrulation |
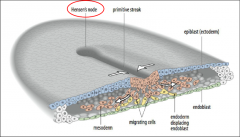
|
|
|
Regression of hensen's node |
Primitive streak regresses, Hensen’s node moves towards the posterior. |
|
|
Where does neurulation begin in the chick? |
•Neurulation begins once the notochord has formed. |
|
|
Early mouse development: cleavage |
Cleavage: cell division produces a blastocyst with a fluid filled blastocoel. Compaction: unique to mammals. Loosely arranged cells become tightly packed. Produces a compacted morula. |
|
|
Two cell types in the mouse blastocyst |
1) Inner cell mass 2) Trophectoderm |
|
|
Inner cell mass |
Inner cell mass give rise to the primitive ectoderm/epiblast and primitive endoderm/hypoblast. |
|
|
Trophectoderm |
Trophectoderm will produce extra embryonic structures. |
|
|
Early post-implantation development of the mouse embryo |
•Mural trophectoderm gives rise to trophoblast giant cells. |
|
|
Basics of mouse gastrulation (similar to gastrulation in chick) |
1) Primitive streak forms in the epiblast at the start of gastrulation. 4) Epiblast cells move through the primitive streak and spread anteriorly and laterally between the epiblast (ectoderm) and the visceral endoderm to form the mesoderm. 7) Some epiblast-derived cells that migrate through the primitive streak displace the visceral endoderm to form the definitive endoderm. Embryo anterior to the node grows rapidly. Node becomes a stem cell center that produces the post-anal tail. |
|
|
Early Embryonic Development |
Cleavage |
|
|
Major consequences of cleavage |
•Each cell division amplifies the total number of cells, but the resulting cells become increasingly smaller with each division during the cleavage phase. |
|
|
Cell Cycle |
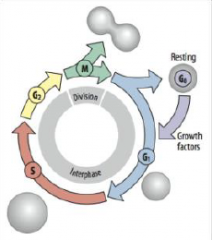
G1 = growth and preparation of the chromosomes for replication |
|
|
Cleavage Cycle |
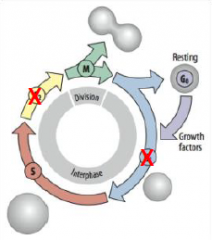
Skips the G1 and G2 growth period between mitotic divisions (biphasic cell cycle). G1 = growth and preparation of the chromosomes for replication |
|
|
Mid-blastula transition (MBT) |
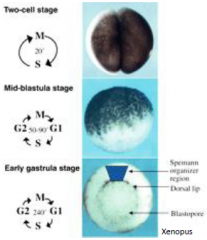
Rapid, synchronous divisions driven by maternal products (RNA and proteins places in the oocyte). At the mid-blastula transition |
|
|
Egg activation and the cleavage cycle |
•Once fertilized, the maternal and paternal genetic material (pronuclei) will fuse, and the now single-celled embryo must divide to produce the cells required to form the multicellular animal. |
|
|
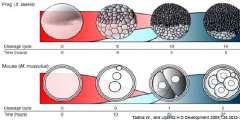
Early development depend on maternal products |
•Maternal RNAs deposited into the egg during oogenesis drive early development. |
|
|
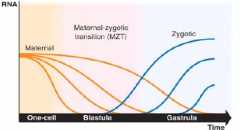
Maternal-Zygotic Transition (MZT) |
•The timing of zygotic genome activation differ across species. |
|
|
Cell division - cytokinesis |
The first visible sign of cytokinesis is when the cell begins to pucker in, a process called furrowing. Furrow is formed by contractile ring. |
|
|
Plane of cleavage – why is this important? |
•Determines the spatial arrangement of daughter cells relative to each other. |
|
|
Determining Plane of Cleavage |
Planes of cleavage are determined by the behaviour of the centrosomes in each cell. |
|
|
Patterns of cleavage |
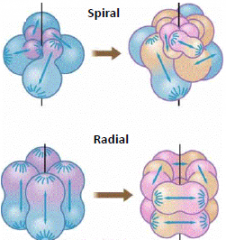
Early protosome embryo. Its four cells are undergoing cleavages oblique to the original body axis: e.g. annelids and molluscs Early deuterostome embryo. Its four cells are undergoing cleavages parallel with and perpendicular to the original body axis. e.g. echinoderms and chordates |
|
|
Types of cleavage |
Amount of yolk affects cleavage - Cleavage furrow extends through the entire egg; entire egg is cleaved. - The entire egg is cleaved but the yolk free portion divides faster than the yolk filled region. - Cleave furrow does not penetrate the entire egg, only the animal pole divides - yolk impedes/halts division. |
|
|
Cleavage in sea urchin |
Sea urchin embryos undergo radial cleavage. |
|
|
Sea urchin blastula |
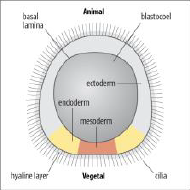
•The blastula is a hollow ball of cells organized into an cilliated epithelial monolayer. |
|
|
Cleavage in mouse |
•Cleavage also occurs in oviduct. First division at ~24 hours and every 12 hours after that to form the morula (~32 cell stage). |
|
|
Mouse blastocyst |
The blastocyst has 2 cell types: Inner Cell Mass and Trophectoderm. |
|
|
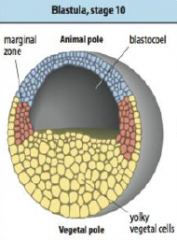
Cleavage in Xenopus |
Egg has distinct polarity, pigmented animal region and a yolky vegetal pole. •First division occurs about an hour after fertilization along the animal-vegetal axis. |
|
|
Xenopus blastula |
|
|
|
Chick blastoderm |
Blastoderm is divided into two areas: 1) the area pellucida (a light area) - area over the subgerminal space and 2) the marginal area opaca (a dark ring). |
|
|
Cells become polarized in the early embryo |
Sea urchin: blastula is a hollow sphere with the outer surface covered in cilia. The single layer epithelium is lined by two extracellular matrices: (i) an inner basal lamina and (ii) an exterior hyaline layer. Cells become polarized in the early embryo. Mouse: embryo undergo compaction, cell-cell contact/adhesion is increased and microvilli become restricted to the apical (outer) surface. |
|
|
Forming the blastocyst inner cell mass (ICM) |
At the 8 cell stage, asymmetric divisions (resulting from a division plane (dashed line) that separates apical and basal domains) produce an inner cell mass cell and a trophectoderm cell, whereas symmetric divisions dividing the apical domain produce two trophectoderm cells. At the 32 cell stage, the position of a cell determines fate, with cells on the inside forming the inner cell mass and cells on the outside taking up a trophectoderm fate. |
|
|
Forming the blastocoel |
Peripheral sealing of the embryo by the formation of adhesive cell-cell tight junctions on the apical side of the epithelium cell layer. Junctions act as a barrier preventing molecules from diffusing across an epithelial sheet between adjacent cells. Mouse: tight junctions begin to form at the 8 cell stage and the barrier is established by the 32 cell stage of the morula. |
|
|
Morphogenesis |
• Processes that bring about changes/rearrangement of cells in the embryo. |
|
|
Morphology of embryonic cells: Epithelial morphology |
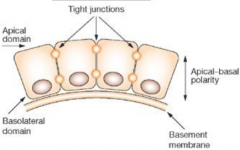
•Apical–basal polarity |
|
|
Morphology of embryonic cells: Mesenchymal morphology |
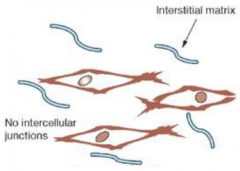
•No distinct polarity |
|
|
Cell Shape |
•Changes in cell shape are generated by the cytoskeleton. |
|
|
Apical constriction |
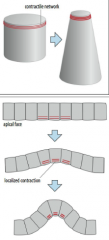
Apical constriction is caused by contraction of a contractile network (composed of actin and myosin) at the apical end of a cell.. |
|
|
Cell Migration |
•Migration takes place over a substratum, e.g. extracellular matrix |
|
|
Cadherins and Integrins |
Cadherins are the main factors adhering embryonic cells. Transmembrane proteins that adhere tightly to the same molecule on other cells in the presence of calcium. Interact with intracellular cytoskeleton via a cytoplasmic tail. Many different types, E-cadherin, N-cadherin, and so on. |
|
|
Differential adhesion |
Separation of cell types with different adhesive properties. |
|
|
The more cohesive ectoderm (epidermis–light blue or grey) remain on the outside... |

...and the less cohesive cells e.g. mesoderm (red) or neural plate (blue) become internalized. -Similar cell types sort and aggregate to each other. |
|
|
Differential Adhesion….. |
…produces a qualitative specificity of adhesion. …provides a mechanism for assembly of different cell types. …stabilize the boundaries between cells. |
|
|
Cadherins aid in …… |
……the separation of distinct tissue layers or the fusion of tissue masses. |
|
|
Morphogenetic Movements: Invagination |
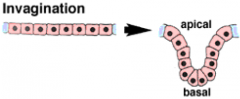
|
|
|
Morphogenetic Movements: Ingression |
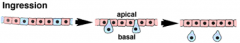
|
|
|
Morphogenetic Movements: Involution |
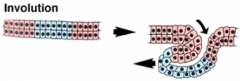
|
|
|
Morphogenetic Movements: Epiboly |
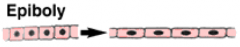
|
|
|
Morphogenetic Movements: Intercalation |
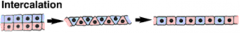
|
|
|
Morphogenetic Movements: Convergent Extension |
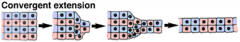
|
|
|
Morphogenetic Movements: Radial Intercalation |
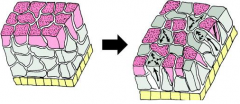
|
|
|
Morphogenetic Movements: Mediolateral Intercalation |
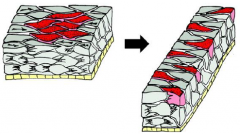
|
|
|
Epithelium to Mesenchymal Transition (EMT) |
Polarized epithelial cell, which normally interacts with basement membrane, undergo multiple biochemical changes that enable it to assume a mesenchymal cell phenotype. EMTs is a mechanisms for…. |
|
|
EMT is a reversible process |
Mesenchymal-to-epithelial transition (MET) involves the conversion of mesenchymal cells to epithelial cells. |
|
|
Movements in Gastrulation: Sea Urchin |
•Ingression of vegetal cells, primary mesenchymal cells, into the blastocoel. |
|
|
Formation of primary mesenchymal cells |
For ingression, primary mesenchyme cells must undergo epithelial to mesenchymal transition to detach from the epithelium of the vegetal plate and ingress into the interior. Changes in their adhesive properties; lose affinity for neighboring epithelial cells and the hyaline layer and gain affinity for the blastocoel wall. |
|
|
Invagination of the vegetal plate |
The vegetal plate undergoes invagination forming the blastpore. Started by apical cytoskeleton contraction of the apical ends of some cells in the vegetal plate, forcing the vegetal plate to buckle. •Primary mesenchyme (mesoderm) cells form skeletal rods (red). |
|
|
Movements in Gastrulation: Chick |
•Begins with the formation of the primitive streak. |
|
|
Primitive streak |
•The primitive forms as a slit/line in the epiblast. |
|
|
Movements in Gastrulation: Mouse |
Early post-implantation development of the mouse embryo |
|
|
Basics of mouse gastrulation (similar to gastrulation in chick) |
1) Epiblast cells move through the primitive streak and spread anteriorly and laterally between the epiblast (ectoderm) and the visceral endoderm to form the mesoderm. 2) Later, the definitive endoderm (derived from epiblast) will replace the visceral endoderm. 3) At the anterior tip of the primitive streak, the node forms (similar to Hensen’s node). 4) Then notochord and somites form anterior to the node. |
|
|
Movements in Gastrulation: Xenopus |
• Invagination (formation of the dorsal blastopore lip) • Involution of the marginal zone (MZ) • Migration of marginal zone cells on the blastocoel roof • Convergent extension of the MZ • Epiboly (spreading) of the ectoderm |
|
|
Formation of the blastopore |
Cells at the MZ undergo apical constriction forming bottle cells. This forces the elongated cell bodies inwards, and creates the dorsal lip and a groove called the blastopore. |
|
|
Involution of mesodermal and endodermal cells |
Presumptive mesoderm and endoderm involute around the blastopore. Involution spreads laterally and ventrally until the blastopore is circular producing the ventral lip. Mesodermal cells migrate anteriorly. |
|
|
Convergent extension elongates the mesoderm |
The dorsal mesoderm elongates in the anterior-posterior direction by convergent extension. |
|
|
Epiboly spreads the ectoderm |
The ectoderm spreads by epiboly to cover the entire surface of the embryo. The animal cap spreads towards the vegetal pole. The individual cells also become smaller and more numerous, through cell division. |
|
|
Molecular Basis of Gastrulation: Mesodermal cell migration |
First mesodermal cells to enter the blastoceol migrate along the blastoceol roof and becomes a part of the anterior/head region. Migratory cells use lamellipodia to migrate along the blastocoel roof as gastrulation proceeds. |
|
|
Fibronectin |
•Large glycoprotein, component of extracellular matrix (ECM) |
|
|
How do migrating mesoderm cells recognize fibronectin? |
-with integrin Integrin • Cell membrane protein. |
|
|
Neutralizing antibody (NAb) |
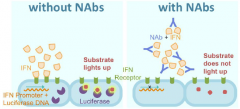
Inhibition of protein function by binding of specific antibodies. |
|
|
Antibodies that specifically block integrin interactions with the RGD of fibronectin were injected into the blastocoel before the onset of gastrulation.... |
....Normal Fibronectin (fn) accumulates along the blastocoel (b) roof. Antibody Injected Block assembly of FN fibrils in ECM. Gastrula shows misplaced mesoderm that is not adherent to the blastocoel roof (H, arrows). No archenteron is present and a centralized remnant of the blastocoel (b) remains. Dorsal (dm) and ventral (vm) mesoderm still evident. |
|
|
What signals and pathways regulate the effectors? |
-Platelet derived growth factor A (PDGF) PDGF signaling is required for directed migration of mesoderm cells across the blastocoel roof. |
|
|
Dominant negative |
The product of a mutated gene adversely affects the function of the wild-type gene. A mutant receptor that, due to dimerization with normal versions of the receptor, has a dominant inhibitory effect upon the normal activity is a dominant negative (dn) mutation. |
|
|
Effects of compromised PDGFA signaling on gastrula. |
Truncated form of PDGF-A mRNA was injected at the four-cell stage into all four blastomeres. Gastrula of uninjected controls (A) are compared with sibling embryos injected with inhibitory dnPDGF-A mRNA (B). Mutant embryo: directional migration by mesodermal cells toward the animal pole is replaced by random migration. |
|
|
Inhibition of PDGFR and PDGF-A results in... |
...diminished head (anterior) structures. |
|
|
Fibronectin, integrin and PDGF signaling are required for... |
...directional migration of mesoderm cells across the blastocoel roof. Mesoderm cells extend lamellipodia towards the animal pole. Cells use integrin to make contact with organized fibronectin in the ECM. PDGF signaling provides directionality in the form of a gradient of PDGF-A signal along the blastocoel roof. The dorsal mesoderm elongates in the anterior posterior direction by convergent extension. |
|
|
Convergent Extension and the Keller Sandwich |
Study of the marginal zone of the amphibian gastrula can be performed using Keller sandwiches. Explants of tissue lying immediately animal to the blastopore lip of an early Xenopus gastrula. Activin treated ectodermal cells behave |
|
|
Protocadherins |
-A factor involved in convergent extension • Similar to cadherins, regulate cell-cell adhesion. |
|
|
Wnt/planar cell polarity (PCP) pathway |
-A factor involved in convergent extension Signaling leads to changes in actin cytoskeleton and cell polarization. Controls lamella formation and myosin contractility Wnt/PCP signaling is needed during gastrulation for efficient convergent extension movements of mesenchymal cells When Wnt/PCP signaling is compromised by loss or gain of function of Wnt/PCP components, the polarized cell behaviors that drive convergent extension movements are perturbed |
|
|
Interfering with Wnt/PCP function... |

...blocks the activin-induced elongation of animal cap cells. The activity of both Protocadherins and PCP signaling are required to coordinate convergent extension movements. |
|
|
Two majors ways to form the neural tube |
1) Primary neurulation - the neural plate cells proliferate, invaginate and pinch off from the surface to form a hollow tube. 2) Secondary neurulation - the neural tube arises from a solid cord of cells that sinks into the embryo and subsequently hollows out to form a hollow tube |
|
|
Neurulation in various vertebrates |
• Fish - exclusively secondary • Birds and mammals - anterior portion of the neural tube - primary - posterior portion of the neural tube - secondary |
|
|
Morphogenetic Processes in Neurulation |
-Columnar thickening of neuroepithelium -Convergent extension of neural plate and underlying mesoderm -Apical wedging of neuroepithelium -Continuing convergent extension -Medial migration of epidermis -Continuing convergent extension -Neural fold fusion -Shaping of lateral/dorsal neural tissue. |
|
|
Neural Tube Closure |
Neural tube closure in the mexican salamander (similar to mammalian), the middle of the tube closes first, followed by both ends. |
|
|
Convergent extension drive neural tube formation |
Wnt/planar cell polarity (PCP) pathway Dishevelled is required for both neural convergent extension and neural tube closure. Inhibition of Wnt/planar cell polarity (PCP) pathway results in neural tube defects. Convergent extension of the neural plate is necessary to reduce the distance between the neural folds, allowing them to meet and fuse. Mediated by differential expression of cell adhesion molecules. |
|
|
Neural tube defects |
Failure to close the tube results in… |
|
|
Totipotent/Pluripotent/Unipotent |
Cells arising from a common lineage (all arising from the zygote) are able to differentiate into different cell types. Totipotent – can give rise to any and all cell types. Pluripotent – can give rise to different cell types. Unipotent – can give rise to a single cell type. |
|
|
Developmental potential |
Developmental potential or potency of a cell describes the range of different cell types it CAN become. |
|
|
Progressive Determination |
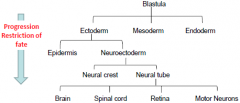
As development proceeds….. |
|
|
Specification map and Fate map |
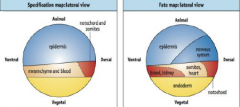
Specification map: describes cell behavior in isolation. Differentiation of explants represents the specification of tissue at time of isolation. Fate map: describes normal development. Does not tell us if cells are specified or determined, i.e. does not indicate developmental commitment. |
|
|
Specification v.s. Fate |
If you get engaged you become specified to someone, but you can change your mind, and you might find someone else and marry them and become determined.
Fate: describes what it will become in the course of normal development. |
|
|
Acquisition of Commitment |
Induction: Cell or group of cells (INDUCER) directs the development of a neighboring cell or group of cells (RESPONDER) -> cell-cell communication. |
|
|
Cell-cell Communication |
Three main ways … |
|
|
Cell-to-cell signaling |
1.Cell (inducer) synthesize signal molecule. |
|
|
PARACRINE (local) SIGNALING |
• Local regulator/signaling molecules are targeted to specific receptors. |
|
|
PARACRINE SIGNALING FACTORS |
• Small diffusible proteins (called ligands). |
|
|
Fibroblast growth factor (FGF) Signaling |
• 25 FGF genes in vertebrates |
|
|
Transforming growth factor beta (TGF-beta) signaling |
• Includes, Activin; Vg-1; bone morphogenic protein (BMP); Nodal and nodal-related. |
|
|
Wingless (Wnt) family signaling – “canonical” Wnt pathway |
• 21 Wnt genes in vertebrates |
|
|
Hedgehog (HH) signaling |
• 3 HH genes in vertebrates (Sonic, Indian and Desert) All bind to same receptor called patched (PTCH) The most common one is the sonic hedgehog gene. |
|
|
Responding to Signals: Competence |
• Competence: the ability of cells to respond to an inductive signal. |
|
|
Acquisition of Commitment |
Induction: Cell or group of cells (INDUCER) directs the development of a neighboring cell or group of cells (RESPONDER) cell-cell communication. |
|
|
Primary Embryonic Induction |
The organizer experiment of Mangold and Spemann (1924) |
|
|
Inductive Event |
Interaction between an inducing cell/tissue that causes a responding cell/tissue to undergo a change in differentiation. |
|
|
Two types of induction |
Instructive induction: the responding tissue has a choice of fates, more than one outcome/response to signaling factors. |
|
|
Morphogenic Gradients |
Cells respond differently to different concentrations of morphogen. |
|
|
Morphogen |
Substance whose spatial concentration differentially directs cell development. |
|
|
How do morphogens have different transcriptional readouts at different concentrations? |
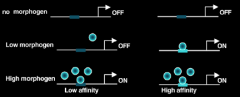
Different targets have different affinities for effectors |
|
|
Criteria for a Morphogen |
1.Secreted molecules - has to form a gradient. |
|
|
Lateral Inhibition |
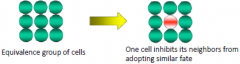
“Telling your neighbors not to be like you” |
|
|
Model for Pattern Formation: Reaction-diffusion Model |
Activator (P) promotes development of cell type A and stimulates production of inhibitor (S). |
|
|
Cytoplasmic Determinants |
Substance located in the cell that guarantees the assumption of a particular state of commitment. |
|
|
The eye has components derived... |
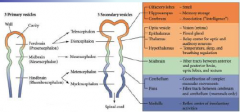
...surface ectoderm, mesoderm and neural ectoderm. |
|
|
Pax6 |
In situ hybridization showing the expression of the Pax6 gene in the developing mouse eye. Pax6 (yellow-green) is expressed in both the presumptive lens ectoderm and optic vesicle/cup (presumptive retina). Pax6 protein is a |
|
|
Eye field specification |
Prospective eye cells are specified prior to evagination of the optic vesicle. |
|
|
Reciprocal Induction |
• 1st optic vesicle induces competence and bias to form lens in the ectoderm. |
|
|
Axis Formation: Xenopus |
•Animal-vegetal pole is related to the anterior-posterior axis. |
|
|
Animal-Vegetal Axis |
• Established by maternal factors. |
|
|
Cortical Rotation |
Cortical cytoplasm rotates 30 degrees during first cleavage – Cortical Rotation. Cortical rotation defines the dorso-ventral axis. |
|
|
Dorsalizing factors |
Specify the location of the dorsal side. Maternal mRNA and proteins localized to the vegetal region. |
|
|
Canonical Wnt signaling pathway |
Without Wnt: |
|
|
Nieuwkoop Center (NC) |
Beta-catenin can induce a new neural axis (dorsal side). |
|
|
Dorso-ventral Axis |
1. Oocyte polarity (Animal-Vegetal) prefigures the germ |
|
|
Four divisions of mesoderm |
1. Axial mesoderm (notochord) 2. Paraxial mesoderm (somites) bone, skeletal muscle 3. Intermediate mesoderm, kidneys, gonads 4. Lateral plate mesoderm, blood, connective tissue, cardiovascular system Animal cells adjacent to vegetal cells become mesoderm not ectoderm. |
|
|
Mesoderm is induced from animal cap cells |
Animal cells adjacent to vegetal cells are induced to become mesoderm. |
|
|
Mesoderm Induction: Competence |
•Tissues at specific times are competent to receive signals. |
|
|
Community Effect |
Cells must be present in sufficient number to be induced. |
|
|
Tissues at specific times are competent to receive signals |
•Animal cap cells competent 4-11 hours after fertilization (7 hour window). |
|
|
Mesoderm Induction: Four Signal Model |
•Signal 1 : originates from the vegetal cells. •Signal 1: General mesoderm inducer, makes "default" ventral mesoderm. |
|
|
Candidate Mesoderm Inducers: Members of TGF-β superfamily |
•Vg1 |
|
|
Is Vg1 required? |
•Maternal transcripts (mRNA) are localized to the vegetal pole in Xenopus. • Vg1 pro-protein has no activity. |
|
|
Is Activin required? |
Activin is sufficient to induce mesoderm in an dose-dependent manner, BUT the mRNA is not detectable. |
|
|
Vg1 vs. Activin |
• Correlation: Activin activates the Nodal signaling pathway (mimics nodal, another TGF-β signaling protein) |
|
|
Xenopus nodal related (Xnr)... |
...proteins turned on in vegetal cells (graded expression) at the appropriate time! |
|
|
Activation of Xenopus nodal related (Xnr) |
•Vegetally localized maternal transcription factor VegT activates the zygotic expression of the Xnrs in the vegetal region. |
|
|
Xenopus nodal related (Xnr) dorso-ventral gradient |
•Low level activation of Xnr by VegT. |
|
|
Signals 3 and 4 pattern the mesoderm dorso-ventrally |
• Dorsalizing signals (3) from the Spemann organizer counteract ventralizing signals (4) from ventral mesoderm. |
|
|
What happens when the tissue from the dorsal side of one embryo (dorsal lip of the blastopore) is transplanted to the ventral side of another embryo? |
Results- This induces cells on the ventral side of the embryo to form a second dorsal axis |
|
|
Noggin is an organizer molecule |
• Expressed in the correct place, the organizer |
|
|
Rescue of dorsal structures by Noggin |
UV radiation -> ventralized embryo |
|
|
Other organizer molecules |
Other organizer molecules, e.g. Chordin and Frizbee, have similar properties as noggin. |
|
|
BMP-4 is a ventral mesoderm inducer |
• Members of TGF-β superfamily |
|
|
How do signals 3 and 4 interact? |
Purified noggin protein binds Model: |
|
|
Four Signal Model |
Signal 1: low level of Xnr Signal 3: Spemann organizer molecules , |
|
|
Mesoderm induction and patterning in chick |
Mesoderm induction and patterning occurs at the primitive streak. Factors that initiate primitive streak formation also signal mesoderm induction and patterning. |
|
|
Antero-posterior Axis |
Once the dorsal side is established, the movement of the involuting mesoderm (gastrulation) establishes the antero-posterior axis. Once the dorsal side is established, the movement of the involuting mesoderm (gastrulation) establishes the antero-posterior axis. |
|
|
Regional Specificity of Induction |
As cells involute over the dorsal blastopore lip during gastrulation, they acquire different fates and inducing properties. |
|
|
Two-inhibitor Model |
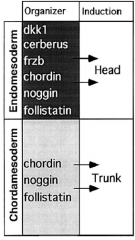
Head induction requires dual inhibition of Wnt and BMP signaling. |
|
|
Head Induction |
Head mesoderm induction requires inhibition of BMP and Wnt signaling. Microinjection of Cerebrus, induces ectopic head. |
|
|
Noggin, a diffusible protein of the organizer |
Noggin is expressed in head mesoderm and notochord. |
|
|
Trunk Induction |
Trunk induction requires inhibition of BMP signaling BUT require Wnt signaling. Block BMP and Xwnt, induce head. Block BMP only, induce trunk. |
|
|
Frizbee, a diffusible protein of the organizer |
Frizbee expressed in head endomesoderm but not notochord. |
|
|
Endoderm Specification |
•Specified by maternal factors in the vegetal region |
|
|
Ectoderm Specification |
Ectodermal determinants are located in the embryo’s animal hemisphere and can direct the differentiation of ectoderm derivatives. |
|
|
Ectodermin (Ecto) specifies ectoderm |
Expression of Ectodermin (Ecto) in Xenopus embryo |
|
|
Morpholinos (MO) |
Morpholinos (MO) are chemically modified antisense oligonucleotides that have a six-membered morpholine-ring backbone. Morpholino oligonucleotides can be designed to bind upstream (5’) of the start codon (AUG) and inhibits progression of the initiation complex, which block translation of the target mRNA. |
|
|
Testing the requirement for Ecto in ectoderm specification |
Ectodermin regulates germ-layer identity along the animal-vegetal axis. Ecto-morpholino (Ecto-MO) (D) and control-MO (C) were injected into one-cell embryos. At the gastrula stage, the mesoderm marker (blue) was upregulated and expanded toward the animal pole in Ecto-MO injected embryos. |
|
|
Ectodermin blocks mesoderm inducing signals |
E3 ubiquitin ligase Ectodermin (Ecto) |

