![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
219 Cards in this Set
- Front
- Back
|
What is a rate?
|
A rate is the change in the property per unit time.
|
|
|
What is a chemical rate?
|
change in concentration of a substance divided by the time it takes that change to occur.
|
|
|
What is a rate law?
|
A rate law is an equation expressing an instantaneous reaction rate in terms of the concentrations, at that instant, of the substances participating in the reaction.
rate = k[A}^x [B}^y |
|
|
What are the exponents in a rate law and what do they mean?
|
x and y are called orders of reaction; x is the order with respect to A and y is the order with respect to B.
These exponents may be integers, fraction, or zero and must be determined experimentally. |
|
|
How do you find the overall order of a rate law?
|
THe overall order of the reaction or reaction order is defined as the sum of the exponents, which is equal to x + y.
|
|
|
What re the three orders of a chemical reaction?
|
Zero-Order, First-Order, Second-Order
|
|
|
In the general reaction:
aA + bB -> cC + dD What does a zero-order reaction look like? Define it. |
Zero-order reactions have a constant rate that is independent of the reactants' concentrations. Therefore the rate law is: rate = k
|
|
|
In the general reaction:
aA + bB -> cC + dD What does a first-order reaction look like? Define it. |
First-order reactions have a rate proportional to the concentration of one reactant.
rate = k[A] or rate = k[B] First order reactions frequently appear in the form of radioactive decay. |
|
|
In the general reaction:
aA + bB -> cC + dD What does a second-order reaction look like? Define it. |
Second order reactions have a rate proportional to the product of the concentrations of two reactants, or to the square of the concentration of a single reactant.
rate = k[A]^2 or rate =k[B]^2, or rate = k[A][B] |
|
|
In order to determine the rate law you need to remember to look for________of reaction trials in which the concentration of only one species changes while the other(s) remain ______
|
pairs, constant
|
|
|
When faced with experimental data what are the steps to finding the rate constant?
|
1: Determine the order of reaction for the each reactant.
2: Find the overall order 3: Plug each order of reaction into a rate law. 4: Find rate constant by using the above rate law and solving for k. Note: use the r-initial when plugging and chugging. |
|
|
How are chemical reactions affected?
|
1) conentration
2) exposed surface area of reactants 3) temperature 4) presence of catalyst. |
|
|
As a rule of thumb the rate of a reaction will approximately ____for every 10C increase in temperature
|
double
|
|
|
How does the medium affect the reaction rate?
|
Certain reactions proceed rapidly in aqueous solution, whereas other reactions may proceed more rapidly in organic solutions, such as benzene. The sate of medium - solid, liquid, gas- can also have a significant effect.
|
|
|
What are the two classifications of catalysts?
|
Homogenous and heterogeneous.
|
|
|
What is a homogenous catalyst?
|
is a catalyst that is present in the same phase as the reactants. So for reactants that are int he gas phase, a homogenous catalyst is also a gas.
|
|
|
What is a heterogeneous catalyst?
|
a catalyst is heterogeneous if it is in a phase different from that of the reactants.
|
|
|
What does a in irreversible reaction mean?
|
the reactions proceed to completion
|
|
|
What does reversible reaction mean?
|
reaction does not go to completion.
|
|
|
Consider the following one step reaction: 2A -><- B + C
What is the rate law for the forwards and reverse reactions ? And what is the equilibrium expression? |
Forward: rate f = kf [A]^2
reverse: rate r = kr [B] + [C] Since rate f and rate r are constants the equilibrium expression is: Kc = [B][C]/[A]^2 |
|
|
What are the characteristics of the equilibrium constant, Kc?
|
1) Pure solids and liquids do not appear in the equilibrium constant expression
2) Large values of Kc (larger than 10^3): the equilibrium favors the products strongly. 3) Intermediate values of Kc (in the range of 10^-3 to 10^3): reactants and products are present in similar amounts at equilibrium. 4) small values of Kc (smaller than 10^-3): the equilibrium favors the reactants strongly. |
|
|
What is the equilibrium expression for:
H2 (g) + Cl2 (g) -><- 2HCl (g) |
Kc = [HCl]^2/[H] + [Cl]
Remember: products/reactants |
|
|
If Kc = 4.0 x 10^31 at 300C, where does the equilibrium lie?
H2 (g) + Cl2 (g) -><- 2HCl (g) |
Which such a large value for Kc (greater than 10^3), the equilibrium lies in favor of HCl, the product side.
|
|
|
WHat is Le Chatelier's principle?
|
Le Chatelier's principle: when a stress is applied to a system at equilibrium, the system will adjust to minimize the effect of the stress.
Remember: no one likes to be stressed |
|
|
What happens when a the concentration of a species is increased?
|
Increasing the concentration of a species will tend to shift the equilibrium away from the species that is added, in order to reestablish its equilibrium concentration, and vice versa.
|
|
|
True or false: In a system at constant temperature, a change in pressure causes a change in volume, and vice versa.
|
True
|
|
|
True or false. Since liquids and solids are virtually incompressible, a change in the pressure or volume of systems involving these phases has a great effect on their equilibrium.
|
False. liquids and solids are incompressible so a change in pressure or volume to those two phases has little or NO effect on their equilibrium.
|
|
|
True or false: Regarding equilibrium, reactions that involve changes in pressure and volume significantly affect gases and have little to no effect on liquids and solids.
|
True.
|
|
|
What is pressure in relation to volume?
|
They are inversely related. An increase in the pressure of a system will shift the equilibrium so as to decrease the number of moles of gas present. This reduces the volume of the system and relieves the stress of increased pressure.
|
|
|
Given N2 (g) + 3 H2 (g) -><- 2NH3 (g) what will happen if:
1) the pressure of the system is increased? 2) the volume of the system is increased? |
The left side has 4 moles and the right side has 2 moles.
1) the equilibrium will shift to the side that has fewer moles of gas. the product side in this case. 2) if volume increased, its pressure immediately decreases, which according to Le Chatelier's principle, leads to a shift in the equilibrium to the left. |
|
|
What must we know before we can understand the changes in temperature have an impact on equilibrium?
Also, describe what would happen if this system was cooled or heated...where would the equilibrium shift? A -><- B + heat |
we need to understand what role heat plays in a chemical reaction. To predict the effect, heat may be considered as a product in an exothermic reaction, and as a reactant in an endothermic reaction.
i.e. A -><- B + heat If this system were cooled, its temp would decrease, driving the reaction to the right, to replace the lost heat. Similarly, if the system were placed in boiling-water, the reaction equilibrium would shift to the left due to the increased concentration of heat. |
|
|
Given the following equation:
CH3)H (l) + H2 (g) -><- CH4 (g) + H2) (l) How would the equilibrium be affected if the volume were doubled? |
The equilibrium would remain unchanged. Changing the volume only affects gases. Furthermore, a volume change only constrains a reaction when one side of the reaction has more moles of gas than the other. In this particular case, the number of moles of gas on each side are equal.
|
|
|
What is the formula for the maximum number of electrons per shell??
|
2n^2.
For example no more than 2(3)^2 = 18 electrons may reside in the n = 3 shell. |
|
|
What is the principal quantum number, n?
|
n, gives the overall energy level and size of the electron's path.
This is also the primary shell # (1,2,3) |
|
|
What is the formula for know the second quantum number or azithumal number (l)?
|
l can be any number 0 to (n-1)
|
|
|
Ture or false. The value of (l) determines the shape of the subshell.
|
True
|
|
|
Which subshell is higher in energy, p or d, assuming the same n value?
|
d.
Only the quantum numbers n and l affect the energy of an orbital. A p orbital has a smaller value of l (l =1) than a d (l=2) orbital does, so the p orbital will be lower in energy than the d orbital. |
|
|
True or false. The higher the l number the higher the energy.
|
True
|
|
|
What is the formula for finding the number of ml values? What does that number give?
|
2l + 1 = the number of orbitals
|
|
|
How many orbitals and electrons are there in the d subshell?
|
Orbitals: use the formula 2l +1.
2(2) +1 = 5 Electrons: use the formula 4l +2 4(2) + 2 = 10 electrons can occupy d subshell. |
|
|
Which is higher in energy, a pz or a py orbital with the same value of n?
|
Neither. pz and py orbitals are equal in energy.
The value of ml does not affect the energy of the orbital --it only dictates the orientation. The pz, py, and px orbitals are degenerate, or equal in energy. |
|
|
What is the Pauli Exclusion Principle?
|
The Pauli Exclusion Principle states that no two electrons in an atom can have the same four quantum numbers.
|
|
|
If n=w, what are the possible quantum numbers that define the depicted atomic electron?
(The picture shows a figure eight with one electron cloud pointing up) n = 2 l = ml = ms = |
n =2
l =1 ml =-1, 0,1 ms = 1/2 or -1/2 |
|
|
What is the rule/formula for determining the order in which subshells are filled?
|
(n+l)
|
|
|
Which will fill first, the 4f subshell or the 5p subshell?
|
4f : 4+3 = 7
5p : 5 + 1 = 6 The 5p because the lowest subshell is always filled first. However 4f has the highest energy level. |
|
|
What does paramagnetic mean?
|
Atoms, ions, or molecules with unpaired electrons.
|
|
|
What does diamagnetic mean?
|
Atoms, ions, or molecules with all paired electrons.
Remember the prefix "di-" in diamagnetic means two. A pair of electrons is two electrons, and all electrons in a diamagnetic species are paired. |
|
|
Is Titanium paramagnetic or diamagnetic?
|
First find the electron configuration.
[Ar] 4s^2 3d^2 The d orbital has two unpaired electrons so it is paramagnetic. |
|
|
What does isoelectronic mean?
|
are atoms, ions, or molecules with an equivalent number of electrons. One of the two isoelectronic pairs will always be an ion.
Take a look at these electron configurations: Ne : [He] 2s^2 2p^6 0^2-: [He] 2s^2 2p^6 Na^+: [He] 2s^2 2p^6 Ne, 0^2- and Na^+ have the same electron configuration, so these species are isolectronic with each other. |
|
|
What are the common names that correspond with the following group Numbers:
I II VII VIII |
I: Alkali Metals
II: Alkaline Earth Metals VII: Halogens VIII: Noble Gases or Inert Gases |
|
|
Which of the following will behave most similar to LiCl?
A. MgCl2 B KCL C. NaH D. BF3 |
KCL because both K and Li are in the same group.
First: look at what groups LiCl are in Second: Look at the answers and find out if they are in the same groups of LiCl. |
|
|
What is the definition of a chemical reaction?
|
a process that converts one or more starting materials into one or more new substances.
|
|
|
WHat is a reactant or reagent?
|
starting material for a reaction (reactants).
|
|
|
What is the first clue you should look for when determining what type of reaction you are looking at?
|
Noting the number of moles of each reactant and product, as well as their respective phases.
|
|
|
WHat are the basic rules for balancing equations?
|
1. Identify the reactants and products of the reaction if they are not completely specified. Write the formula(s) of the reactants on the left side of the arrow and the formula(s) of the products on the right side of the arrow.
2. Balance the element that appears in the fewest number of molecules. 3. Continue balancing the elements as they appear. Balance las the element that appears in the most number of molecules. 4. As necessary, multiply all coefficients on both sides of the reaction by the same amount in order to clear fractional coefficients. |
|
|
What are the two categories for classifying reactions? give examples of each.
|
1) classification based on the compositions or reactants and products, (i.e. combination and decomposition reactions)
2) classification based on the driving force of the reaction. (i.e. single displacement and double displacement reactions. |
|
|
What are combination reactions? What are specific types of combination reactions?
|
combination reactions are reactions in which two or more reactants combine to form one product.
i.e. A +B -> AB combustion, formation, corrosion reactions |
|
|
What are combustion reactions?
What are some trends? |
The combustion reaction is a type of combination reaction, where a substance is burned in the presence of a gas (g).
i.e. Magnesium burns in the presence of nitrogen gas to form solid magnesium nitride: 3Mg (s) + N2 (g) -> Mg3N2(s) Sodium burns in the presence of chlorine gas to form solid sodium chloride: 2Na(s) + Cl2(g) -> 2NaCl (s) Sulfur burns in the presence of oxygen to form sulfur dioxide: S(s) + O2(g) -> SO2 (g) These three reactions have several things in common. First, there are two reactants combining to make one product, making each reaction a combination reaction. Also, in each reaction, of the reactants is a gas, making each reaction a combustion reaction. |
|
|
What are formation reactions are what general reaction type are they?
|
Formation reactions are types of combination reactions in which a single product is formed from elements in their standard states.
Examples: Gaseous propane can be formed from its elements in their standard states: 3C (s, graphite) + 4Hx(g) -> C3H8 (g) Carbon dioxide gas can be formed from its elements in their standard states C (s, graphite) + )2(g) -> CO2(g) THings in common: they both have a generic formula for combination reactions A + B -> AB. Also, each of the reactants is an element in its standard state. THe standard state is defined as the phase or state, an element is in at one atm. |
|
|
What are corrosion reactions and what general reaction type are they?
|
metals deteriorate in corrosion reactions when a liquid or gas, usually oxygen, chemically attacks the surface of the metal.
THe most common example of corrosion is the rusting of iron: 4Fe(s) + 3)2(g) -> 2Fe2O3(s) |
|
|
True or false: The following reaction is a corrosion reaction:
C(s, graphite) + O2 -> CO2 (g) |
False
While the reaction of a graphite with oxygen may initially appear to be similar to a corrosion reaction, the def. of a corrosion reaction requires a metal to be one of the reactants. Graphite is a non-metal. |
|
|
What are decomposition reactions?
|
Decomposition reactions occur when a compound breaks down into two or more substances, usually as a result of heating, electrolysis, or light.
Generic formula: AB -> A + B |
|
|
What are some symbols that can be placed above the arrow of the chemical equation to depict the method of decomposition?
|
Delta triangle, UV
|
|
|
What types of reactions depict :
A +B -> AB ? |
combustion, formation, corrosion
|
|
|
What types of reaction depicts :
AB -> A + B ? |
decomposition
|
|
|
Another type of reaction classification is the driving force of the reaction. What are some examples?
|
Among these forces are:
1) an increase in entropy due to gas formation 2) transfer of electrons to achieve greater stability, 3) formation of a solid precipitate. |
|
|
What are some category examples of driving force reactions? What do each include?
|
single displacement reactions and double displacement reactions.
Single displacement reactions include reduction-oxodation reactions. Double displacement reactions include neutralization and precipitation reactions. |
|
|
What is a single displacement reaction?
|
they occur when an atom (or ion) of one compound is replaced by an atom of another element.
Generic formula: A + BC -> AB + C Zn(s) + CuSO4(aq) -> ZnS)4(aq) + Cu(s) Single displacement reactions are often further classified as redox reactions. |
|
|
What is a redox reaction and how do you determine if it is?
|
In a redox reaction, electrons are transferred between species. In order to determine if electrons have been transferred among reactants, it is necessary to recognize the oxidation states, or oxidation numbers, of the reactants and products.
|
|
|
What is an oxidation number?
|
The oxidation number is the number assigned to an atom in an ion or molecule that denotes its real or hypothetical charge.
|
|
|
How do you assign oxidation numbers? IMPORTANT
|
1) The oxidation number of free element is 0
2) The oxidation number of a monatomic ion equals the charge of the ion. 3) The sum of the oxidation numbers in a neutral compound is 0. 4) For a polyatomic ion, the sum of the oxidation numbers equals the overall charge of the ion. 5) The more electronegative element in a species assigned its typical negative oxidation number; the more electropositive element has a positive oxidation number. 6) Fluorine, the most electronegative element, always has a -1 oxidation state in its compounds. Oxygen, second only to fluorine in electronegativity, generally has an oxidation state of -2 in its compounds; the two exceptions are in peroxides, where the oxidation state of oxygen is -1, and the superoxides, where it is -1/2. Hydrogen usually has an oxidation state of +1, except when paired with more electropositive elements, which make it have an oxidation state of -1. |
|
|
WHat is the oxidation state of the following examples?
H2 Fe^3+ I^1- HCl LiH |
H2 = oxidation number is 0 because it is a free element
Fe 3+ = oxidation state is 3+ because the oxidation number of a monatomic ion equals the charge of the ion. i^-1 = oxidation state is -1 because the oxidation number of a monoatomic ion equals the charge of the ion - HCL = H has an oxidation state of 1+ and Cl has an oxidation state of -1 b/c Cl is more electronegative and takes it typical oxidation number. LiH, Li is 1+ and H is -1 b/c hydrogen is more electronegative than lithium and takes the negative charge. |
|
|
What is the oxidation number of nitrogen in nitric acid, HNO3?
|
+5.
In neutral molecules, the sum of all the oxidation numbers must equal zero. In HNO3, the H has an oxidation state of +1, and ) has an oxidation state of -2. As there are three oxygen in the molecule, the total charge of oxygen is -6. Nitrogen, therefore, must have an oxidation state of +5 to make the sum of the oxidation numbers equal to zero. |
|
|
What are Redox reactions?
|
Reduction-oxidation, or redox, reactions involve the transfer of electrons from one species to another.
|
|
|
True or false? In a redox reaction, the oxidation number of at least one element will change?
|
True
|
|
|
Another type of reaction classification is the driving force of the reaction. What are some examples?
|
Among these forces are:
1) an increase in entropy due to gas formation 2) transfer of electrons to achieve greater stability, 3) formation of a solid precipitate. |
|
|
What are some category examples of driving force reactions? What do each include?
|
single displacement reactions and double displacement reactions.
Single displacement reactions include reduction-oxodation reactions. Double displacement reactions include neutralization and precipitation reactions. |
|
|
What is a single displacement reaction?
|
they occur when an atom (or ion) of one compound is replaced by an atom of another element.
Generic formula: A + BC -> AB + C Zn(s) + CuSO4(aq) -> ZnS)4(aq) + Cu(s) Single displacement reactions are often further classified as redox reactions. |
|
|
What is a redox reaction and how do you determine if it is?
|
In a redox reaction, electrons are transferred between species. In order to determine if electrons have been transferred among reactants, it is necessary to recognize the oxidation states, or oxidation numbers, of the reactants and products.
|
|
|
What is an oxidation number?
|
The oxidation number is the number assigned to an atom in an ion or molecule that denotes its real or hypothetical charge.
|
|
|
How do you assign oxidation numbers? IMPORTANT
|
1) The oxidation number of free element is 0
2) The oxidation number of a monatomic ion equals the charge of the ion. 3) The sum of the oxidation numbers in a neutral compound is 0. 4) For a polyatomic ion, the sum of the oxidation numbers equals the overall charge of the ion. 5) The more electronegative element in a species assigned its typical negative oxidation number; the more electropositive element has a positive oxidation number. 6) Fluorine, the most electronegative element, always has a -1 oxidation state in its compounds. Oxygen, second only to fluorine in electronegativity, generally has an oxidation state of -2 in its compounds; the two exceptions are in peroxides, where the oxidation state of oxygen is -1, and the superoxides, where it is -1/2. Hydrogen usually has an oxidation state of +1, except when paired with more electropositive elements, which make it have an oxidation state of -1. |
|
|
WHat is the oxidation state of the following examples?
H2 Fe^3+ I^1- HCl LiH |
H2 = oxidation number is 0 because it is a free element
Fe 3+ = oxidation state is 3+ because the oxidation number of a monatomic ion equals the charge of the ion. i^-1 = oxidation state is -1 because the oxidation number of a monoatomic ion equals the charge of the ion - HCL = H has an oxidation state of 1+ and Cl has an oxidation state of -1 b/c Cl is more electronegative and takes it typical oxidation number. LiH, Li is 1+ and H is -1 b/c hydrogen is more electronegative than lithium and takes the negative charge. |
|
|
What is the oxidation number of nitrogen in nitric acid, HNO3?
|
+5.
In neutral molecules, the sum of all the oxidation numbers must equal zero. In HNO3, the H has an oxidation state of +1, and ) has an oxidation state of -2. As there are three oxygen in the molecule, the total charge of oxygen is -6. Nitrogen, therefore, must have an oxidation state of +5 to make the sum of the oxidation numbers equal to zero. |
|
|
What are Redox reactions?
|
Reduction-oxidation, or redox, reactions involve the transfer of electrons from one species to another.
|
|
|
True or false? In a redox reaction, the oxidation number of at least one element will change?
|
True
|
|
|
What are the oxidation states for each atom and molecule? Is this a redox reaction?
3Mg(s) + N2(g) -> Mg3N2(s) |
3Mg(s) = 0
N2 (g) = 0 Mg3N2(s) = (Mg3: +2, N2: -3) Notice that both side have a total of zero. Mg = 3 x +2 = +6 e- and N2 = 2 x -3 = -6 e-. so +6 and -6 = 0 |
|
|
Which one is oxidized and reduced? Which one is the reducing agent and oxidizing agent?
3Mg(s) + N2(g) -> Mg3N2(s) |
3Mg(s) = 0
N2 (g) = 0 Mg3N2(s) = (Mg3: +2, N2: -3) Oxidized = Mg3 Reduced = N2 Oxidizing agent = N2 Reducing agent = Mg3 |
|
|
What is an oxidizing agent?
What is a reducing agent? |
a substance that accepts electrons from another species.
A substance that donates electrons to another species. |
|
|
What are double displacement reactions? What are specific types of displacement reactions?
|
Also called metathesis reactions, elements from two different compounds displace each other to form two new compounds.
Generic formula: AB + CD -> AC + BD Example: CaCl2(aq) + 2AgNO3(aq) -> Ca(NO3)2(aq) + 2AgCl(s) Neutralization and precipitation reactions. |
|
|
What is a neutralization reaction?
|
involves an acid and a base reacting to form a salt and usually water.
Examples: NaOH(aq) + HCl(aq) --> NaCl(aq) + H2O(l) another example: Ba(OH)2(aq) + 2HCl(aq) --> BaCl2(aq) + 2H2O(l) Each example shows an acid reacting with a base. According to the Bronsted-Lowery def of acids bases, an acid is a proton donor and a base is a proton acceptor. The Lewis def. defines an acid as an electron pair acceptor, while a base is an electron pair donor. |
|
|
What is the Bronsted-Lowry of an acid and base?
|
Acid = proton donor (O+)
Base = proton acceptor (O:-) |
|
|
What is the Lewis definition of an acid and base?
|
Acid = electron pair acceptor (O+)
Base = electron pair donor (O:-) |
|
|
What type of acid and base is it when there is no water formed in a neutralization reaction?
|
This type of reaction occurs when the acid or the base is a Lewis but not Bronsted-Lowry.
Two examples are: BF3 + NaOH --> NaB(OH)F3 NH3 + HCl --> NH4Cl Notice, however, that these example are NOT double displacement reactions, although they are neutralization reactions. |
|
|
On test day if you see titration reactions they will most likely be...
|
neutralization reactions.
|
|
|
What are precipitation reactions?
|
Precipitation reactions are a specific type of metathesis (double displacement) reaction in which a solid product forms.
Example: NaOH(aq) + AgNO3(aq) --> NaNO3(aq) + AgOH(s) |
|
|
WHat is the over all reaction of NaOH (aq) + AgNO3(aq) --> NaNO3(aq) + AgOH(s)
|
Na+(aq) + OH-(aq) + Ag+(aq) + NO3-(aq) --> Na+(aq) + NO3-(aq) + AgOH(s).
Na+ and NO3- are actually spectator ions. That is why we did not sperate AgOH(s). Over all reaction is therefore: Ag+(aq) + OH-(aq) --> AgOH (s) Although the overall reaction appears to follow the formula for a combination reaction, the reacting ions are from two different species, hence it is still a double displacement reaction. |
|
|
On test day if you see text about solutions and solubility you will most likely see a ____reaction.
|
precipitation. (salts/solids)
|
|
|
This reaction is what type general and specific type of reaction?
A + BC --> AB + C |
Single displacement Reactions
Redox |
|
|
This reaction is what type general and specific type of reaction?
AB + CD --> AC +BD |
Double Displacement Reactions
Neutralization and Precipitation |
|
|
This reaction is what type general and specific type of reaction?
A + B --> AB |
Combination Reactions
Combustion, Formation, Corrosion |
|
|
This reaction is what type general and specific type of reaction?
AB --> A + B |
Decomposition Reactions
|
|

WHat is this question ultimately asking for?
|
The Delta H of a reaction = DelatHformation(products) - DeltaHformation(reactants)
Remember any monoatomic ion has a deltaHformation of 0 |
|

What does this yield and why?
|
yields CO2 and H20 as products.
The complete oxidation of any hydrocarbon (or carbohydrate) composed solely of carbon, hydrogen, and oxygen is a combustion reaction leading to the products CO2 and H2O. |
|
|
What atoms normally go through hydrogen bonding?
|
Hydrogen bonding occurs when a hydrogen atom is bonded to F, O or N
|
|
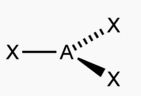
What shape is this and its bond angles?
|
Trigonal Planar; All angles 120 degrees
|
|
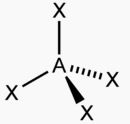
What shape is this and what are the bond angles?
|
Tetrahedral; All 109.5
|
|

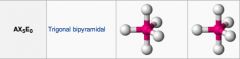
What shape is this and what are the bond angles? How many things does it have on central atom?
|
Trigonal bipyramid: there are 90 and 120 angles.
5 things |
|

What shape is this and what are the bond angles? How many things does it have on central atom?
|
Octahedral (hedral meaning faces): all angles are 90 degrees
6 things |
|
What geometric shape do these molecules have?
|

|
|
What geometric shape do these molecules have?
|

|
|

What geometric shape do these molecules have?
|
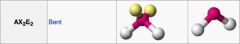
|
|

What geometric shape do these molecules have?
|
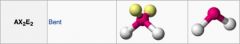
|
|
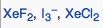
What geometric shape do these molecules have?
|

|
|
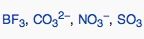
What geometric shape do these molecules have?
|
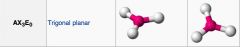
|
|
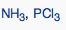
What geometric shape do these molecules have?
|
|
|
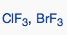
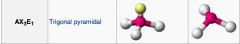
What geometric shape do these molecules have?
|

|
|

What geometric shape do these molecules have?
|
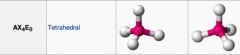
|
|

What geometric shape do these molecules have?
|

|
|
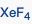
What geometric shape do these molecules have?
|
|
|

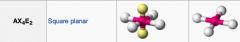
What geometric shape do these molecules have?
|
|
|

What geometric shape do these molecules have?
|
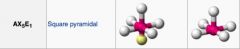
|
|

What geometric shape do these molecules have?
|
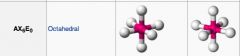
|
|

What geometric shape do these molecules have?
|
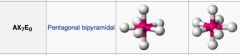
|
|
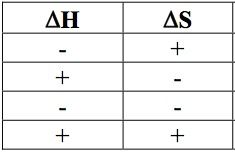
What are the results of the above scenarios?
|

|
|

Are these at equilibrium, spontaneous, or non-spontaneous.
|

|
|

Are these at equilibrium, spontaneous, or non-spontaneous.
|

|
|
|
What are the rules for oxidation numbers?
|
1) Elements in their elemental form are in the zero oxidation state.
2) Group 1 metals are +1 and Group 2 metals are +2. 3) Hydrogen is +1 except when bonded to metals (when it's -1) 4) Transition elements must be determined by context; (except: Al = +3, Zn = +2, Cd = +2, Ag = +1) 5) The most electronegative elements get their typical oxidation state. 6) The last element not assigned balances the charge of the compound/ion. |
|
|
What are the characteristics of Electrochemical Cells?
|
a) The anode is always the site of oxidation (an ox)
b) The cathode is always the site of reduction (red cat) c) Electrons always flow from anode to cathode d) Anions flow to the anode and cations to the cathode through the salt bridge e) In the galvanic/voltaic (spontaneous) cells, the cathode is + and the anode is -; The sings are the opposite in electrolytic (non-spontaneous) cells. f) For metal/metal salt solution cells, the cathode gains mass, while the anode loses mass. |
|
|
What are some examples of elements in their elemental form having an oxidation state of 0?
|
O2 (diatomic), Na (monatomic), O3, S8 (even though there are 8 S they are still one element)
|
|
|
What are the intermolecular forces trends for:
B.P. M.P. Vapor Pressure Viscosity Surface Tension What bond trumps all of the forces in relation to strenght? |
B.P & M.P..: increase in forces = increase in B.P. and M.P.
Vapor Pressure: High Molecular Forces = Lower Vapor Pressure Viscosity: - High Molecular Force = High Viscosity Surface Tension - HIgh Molecular Force = High Surface Tension Donald Trump of them all = Ionic Bonds (ie. Na-Cl) |
|
|
When going from a solid -> liquid -> gas, is this endothermic or exothermic?
2) What is Delta H? 3) What is Delta S? When going from a gas -> liquid -> solid, is this endothermic or exothermic? 2) What is Delta H? 3) What is Delta S? |
solid -> liquid -> gas,
1) Endothermic 2) Delta H (+) > 0 3) Delat S (+) > 0 (increase in disorder) gas -> liquid -> solid, 1) Exothermic (Bonds formed releases energy) 2) Delta H (-) < 0 3) Delta S (-) < 0 (decrease is disorder) |
|
|
When dealing with gases what must the units always be?
For gases do we use STP or Standard Conditions? 1 mole of gas is equal to how many Liters? What is the ideal gas law? |
Temp = Kelvin (always)
Volume - L We use STP T = 273 K P = 1atm 3) 1mole gas/ 22.4 L P = nRT |
|
|
How would you find know if something behaved like an ideal gas?
|
Find the molecule that has the least amount of attractive forces with each other and behaves drastically.
|
|
|
If you have more liters will you have more or less moles?
Which one has more moles 10 L vs. 20 L |
More Liters = More Moles
20L has more moles |
|
|
According to CHad what is diffusion?
What example did CHad use when talking about Effusion? |
1) Diffusion = high concentration -> low concentration
2) Effusion = think of a balloon with small hole. => effusion is when gases pass through a narrow slit. => Grahams's law talks about which one will escape faster. Remember: For both Effusion and Diffusion the lightest molecule will travel the fastest. |
|
|
What are the four BIG colligative properties?
|
1) Freezing Point Depression
2) Boiling Point Elevation 3) Vapor Pressure Depression 4) Osmotic Pressure |
|
|
If you increase the solutes what will happen to the freezing point?
Does it matter what type of solutes you have? |
Increase solute = decrease in freezing point
-No, It doesn't matter what type of solute you have. The quantity dumped in matters. |
|
|
What two options do you have to make a compound boil?
|
1) increase temperature or..
2) Decrease the external Vapor Pressure (i.e.on top of mount Everest. means that less pressure is keeping the molecules in their liquid state) |
|
|
What is diffusion?
|
Movement of solute from high concentration to low concentration.
|
|
|
True or false. In osmotic pressure water moves to the most concentrated/impure area?
|
True. Water moves from an area of low concentration to high concentration.
When dealing with osmosis focus only on where water wants to go...not the concentration of solutes. |
|
|
What is the difference between Kinetics and Thermodynamics?
|
Kinetics can tell us:
- Only the speed Thermodynamics can tell us if: - a reaction is spontaneous - endo or exo thermic - entropy increases or decreases - Kc(concentration) shifts left or right. - BUT it can't tell us how fast |
|
|
Diamond or Graphite, which one is more stable?
|
Notice that I didn't ask which one was more stronger?
Graphite is more stable b/c of its standard state of C. |
|
|
WHat is Delta H
|
Enthalpy
Delta H = products - reactants -Delta H = exothermic +Delta H = Endothermic |
|
|
What does and doesn't show up in equilibrium constants?
|
Does show up:
Aqueous (aq) Gases Does NOT show up: solids liquids |
|
|
What is the only thing that can change Keq
|
Changing the temperature
|
|
|
WHat will happen to the equilibrium if you add an inert gas?
|
Nothing
|
|
|
If I told you that the Pressure increased and the volume decreased but the Eq did not shift? WHat happened
|
Same # of mole of Reactants and Products
|
|
|
True or false? The solubility Equilibrium expression, for the following rxn, is:
AgCl (s) -> Ag+(aq) + Cl-(aq) Ksp = [Ag+] [Cl-] / [AgCl) |
False! Remember: Regardless of what is in front of the K (Keq, Ka, Kb, Kc, Kp, Ksp), K is still dealing with equilibrium. Which means. There are only two that that are shown and not shown
Shown: gas aq Not Shown: Solids liquids AgCl (s) is a solid. It won't show up. So Ksp = [Ag+] [Cl-] |
|
|
What is molar solubility?
|
the amount of solid that dissolves. It is the molar concentration of solid that dissolves.
|
|
|
What are the three types of acids and bases?
|
Arrhenius
Bronsted/Lowry (The "o" in Bronsted and Lowry means that we will focus on pr-"o"-tons. Lewis (The "e" in Lewis means he focuses on electrons) |
|
|
Describe how each these types define acids and bases.
Arrhenius Bronsted/Lowry Lewis |
Arrhenius:
As Acid: increases [H+] in H20 As Base: increases [OH0] in H20 Bronsted/Lowry As Acid: Proton (H+) donor As Base: Proton (H+) acceptor Lewis: As Acid: Electron (e-) acceptor pair As Base: Electron (e-) donor |
|
|
What is an amphoteric substance?
|
a substance that acts as an acid in some reactions and a base in others.
i.e. Water can act as an acid or base. |
|
|
What are the 7 strong acids that you should memorize.
This is the acid roster. If an acid is not on this list then...IT IS NOT A STRONG ACID...NO MATTER WHAT!!! |
HCl
HBr HI H2SO4 HNO3 HClO4 HClO3 |
|
|
What are the Strong Bases?
What are the most common weak bases? |
LiOH
NaOH KOH RbOH CgOH Ca(OH)2 Sr(OH)2 Ba(OH)2 Common Weak bases? NH3 Amines |
|
|
What is the trend for binary acid strength?
|
binary acid = those containing only hydrogen and a nonmetallic element; i.e. HA
The larger the molecule A is, the stronger the acid will be. -> As you move down the periodic table molecules get larger The more polar the bond between H and A is, the stronger the acid will be. -> As you from left to right on the periodic table b/c of electronegativity. |
|
|
What are the trends for oxoacid strength?
|
Oxoacid-acids that contain oxygen
Compare the conjugate base - Weak conjugate base = strong acid - Weak base is weak by being stable - A base is stable through through resonance. More stable bases move up the periodic table. So the periodic trend for oxoacids is opposite to that of binary acids . -> acid strength increase up the periodic table with oxoacids b/c the conjugate base is more stabilized by electronegativity. |
|
|
WHat is the relationship between the the two ions [H+] and [OH-]?
If Neutral Acidic Basic |
Neutral: [H+] = [OH-]
Acidic [H+] > [OH-] Basic: [H+] < [OH-] |
|
|
WHat does p stand for?
What does pH stand for? |
"p" is just a way for chemists change exponents into log language.
THus, "p" = -log (always assume base 10) pH = means the power of hydrogen ion concentration in a solution pH = -log[H+] |
|
|
Know these basic trends of logorithms
log(0.01) = ? = ? log (0.1) = ? = ? log (1) = ? = ? log (10 = ? = ? log (100) = ? = ? |
log(0.01) = log10^-2 = -2
log (0.1) = log10^-1 = -1 log (1) = log10^0 = 0 log (10) = log10^1 = 1 log (100) = log10^2 = 2 |
|
|
K, for any problem, is what?
|
K = the expression that describes the equilibrium of a reaction where the concentrations of the materials are known as K.
Remember solids and liquids are not shown. But gases and aqueous (aq) are shown. |
|
|
Kc =
Kp = |
Kc = The equilibrium constant, Kc, is used to describe the concentrations of reactants and products at equilibrium.
Kp = The equilibrium constant, Kp, is good to use when the reactants and products are gases. The subscript, "p", represents pressure. In this expression, the equilibrium is described in terms of the partial pressures of the reactants and products. aA + bB -> cC + dD Kp = (PC)^c + (PD)^d ________________ (PA)^a + (PB)^b |
|
|
What is the relationship between Kc and Kp?
|
Well if you find the one you can usually find the other, through the PV = nRT rule.
Kp = Kc(RT)^Delta n Kc = Kp (1/RT)^Delta n |
|
|
WHen do you use
Ka and Kb |
When you are faced with a weak acid or weak base.
|
|
|
If the acid or base can you find the pH, pOH, [H+] and [OH+] EXACTLY?
What if is a strong acid/base but with an extra H or OH What if it is a weak acid/base? |
YES
Then it will slightly less than the exact amount of the first dissociated H (ie. HCl vs. H2SO4) You must use Ka or Kb if it is a weak acid or base (Not on the "roster sheet" of strong acids and bases. Everything else is weak and must use Ka and Kb) |
|
|
WHat is the equilibrium expression for Ka and Kb? Why?
|
Ka = [H+] [A-]
_______ [HA] The same is for Kb, except replace [H+] with [OH-]. The reason the [HA] is on the bottom is because it is aq. |
|
|
What thing maintains pH and resists changes to pH?
How do you create a buffer? |
A buffer.
Weak Acid + Conjugate Base Weak Base + Conj. Acid Know your salts will help you with this. |
|
|
WHat are the three ways to make a buffer?
|
1) -approx 1 equivalent WA per 1 equivalent CB
2) 1 equivalent WA per 1/2 of Strong Base (SB) 3) 1 equivalent of WB per 1/2 equivalent of strong acid. |
|
|
WHat is Acid Base Titration?
|
is a procedure that is used where a base of known concentration is added to an acid of unknown concentration (or visa versa) in order to determine the concentration of the unknown
|
|
|
What are the three laws of thermodynamics.
|
1) Energy can neither be created nor destroyed. It is simply exchanged between the system and the universe.
2) For a spontaneous process, the entropy of universe increases. 3) States that the entropy of a pure solid at absolute zero (0K) = zero entropy. It is as cold as it can go. |
|
|
What is the definition of a state function and why is this important to know?
Give examples of who is a state function: |
State function is one the depends only on the initial and final state of the system. It does not focus on the pathway.
We are only worried about the beginning and the end of the journey, not how they got there = state function. Delta E Delta H Delta S Delta G |
|
|
Who is a state function here and who is not:
Delta E = q + w |
Delta E is a state function
q and w are not. Their pathways matter. |
|
|
If a system gains heat what does q =
If a system loses heat what does q equal? |
system gains heat = +q
system loses heat = -q |
|
|
Quickly define all of these
Delta G Delta H Delta S |
delta G= delta H - T(deltaS)
delta G = change in gibbs free energy delta H = change in enthalpy delta S = change in entropy T = temperature at which the rxn is occuring. |
|
|
What is the equation for measuring heat of a reaction?
|
q = mcDeltaT
m = mass in grams c (or s) = specific heat (the amount of heat required to raise 1 gram of the substance 1C) |
|
|
When we talk about work what phase are we usually referring to?
|
Gas
|
|
|
As gas expands what sign is w?
As gas compresses what sign is w? |
gas expands = -w (loses energy)
gas compresses = +w ( gains energy) (The universe is doing work on the gas, giving the gas more energy) |
|
|
What is the single biggest indicator when looking at the change in entropy?
|
focus on the moles of gases.
the 2nd is look at the phase changes. |
|
|
WHat is the difference between q and Delta H?
|
Delta H is a state function and q is not (pathway matters with q)
|
|
|
There are three ways to solve for enthalpy. How do you know to use one of the three ways?
|
DAT gives you: Enthalpies of formation
- use DeltaHrxn = products - reactants DAT gives you bond energies: - DeltaHrxn = Broken (reactants) - Formed (products) DAT gives you Hess's law - sucks for you :). But you will do it! |
|
|
What is Delta Hformation?
|
Formation rxns are very specific. They form 1 mole from its individual elements in their standard states
|
|
|
What type of molecule will always have a heat of formation of 0?
|
Diatomic molecuels
|
|
|
What is the mnemonic for knowing the diatomic molecules?
|
Never Have Fear Of Ice Cold Beer
N= nitrogen H = Hydrogen F = Flourine O = Oxygen I = Iodine C = chlorine Br = Bromine or remember they make the number 7 on the periodic table |
|
|
What are the only two elements on the periodic table that are liquid in their standard state?
|
Hg and Br
|
|
|
Are all metals solids?
|
No, Hg
|
|
|
WHen dealing with formation reactions, if i tried to balance out the reaction by multiplying across to get ride of the fractions would it still be considered a Formation Rxn?
|
Noooo! b/c that would change their standard elemental state. They need to remain in their elemental form.
|
|
|
Do we have reference points for Delta G, Delta H and Delta S?
|
Not for Delta G and Delta H. But we do have a reference point for Delta S.
0 degrees Kelvin. |
|
|
What is the difference between Standard conditions and STP
|
Standard Conditions
P = 1 atm T = 25C = 293K All concentrations are = 1M or 1 ATM STP P = 1 atm T = 0C = 273 K Use Standard Conditions when dealing with thermodynamics Use STP (PV=nRT) when dealing with GASES. |
|
|
In Standard Conditions which one do you have more of products or reactants?
|
Both are the same b/c "all concentrations are equal to 1M or 1 ATM in standard conditions
|
|
|
What image do you think of when Delta G = 0
|
A skateboard dude at the bottom of a half pipe. NO more potential energy.
Delta G has reached equilibrium = 0 |
|
|
What do the + and - symbols mean for Delta G in standard conditions?
|
-Delta G <0 = spontanteous = more products = Keq > 1
+Delta G >0 = non-sponteaneous = more reactants = Keq < 1 Delta G = 0 =neither = equal reactants and products = Keq = 1 |
|
|
What are the solubility rules for things that are soluble?
|
Soluble compounds:
- All compounds of alkali metals (group 1A) are soluble - All salts containing NH4+, NO3-, ClO4-, and C2H3O2- (acetate) = soluble - All chlorides, bromides, and iodides (salts containing Cl-, Br-, or I-) = soluble EXCEPTION (to the above): Halides where Ag+, Pb2+, Hg2^2+, are the cations are insoluble -All sulfates (salts containing SO4^2-) are soluble EXCEPTION (to the above): Sulfates with the cations Hg2+, Pb2+, Ca2+, Sr2+, and Ba2+ = insoluble |
|
|
What are the solubility rules for things that are not soluble?
|
insolubility rules + exceptions:
- All hydroxides (-OH comounds) and all metal oxides (O^2- compounds) = insoluble EXCEPTION (to the above): Hydroxides and metal oxides combined with group 1A elements and CA^2+, Sr^2+ and Ba^2+ = soluble And, another exception, Group 1A and NH4+ compounds are soluble. NOTE: when metal oxides do dissolve, they react with water to form hydroxides For example: Na2O(s) + H2O(l) --> 2NaOH(aq) - All compounds that contain PO4^3-, CO3^2-, SO3^2-, and S^2- = insoluble |
|
|
What is the chemical formula for acetic acid?
|
CH3CO2H = monoprotic
|
|
|
What usually forms acidic anhydrides and basic anhydrides?
|
Metals usually form basic anhydrides
non-metals usually form acidic anhydrides. |
|
|
What is vapor pressure?
|
Vapor pressure is the amount of pressure the gas phase of a substance exerts over the liquid phase.
The vapor pressure of a liquid is affected by temperature and pressure, as well as by the amount of solute dissolved in the liquid. - Changing the amount of liquid will have not effect on the ability of liquid molecules to become gas molecules. THus the vapor pressure will remain the same. |
|
|
How do catalysts generally operate?
|
They operate by lowering the activation energy of a reaction pathway (and thus changing the kinetics of the reaction) without affecting the thermodynamics of the reaction.
|
|
|
Will a catalyst affect the Delta G?
|
No. Delta G is a thermodynamic value. Catalysts only affect kinetics/activation energy.
|
|
|
True or false. A catalyst will lower the activation energy for both forward and reverse reactions?
|
True. The activation energy is lowered in both directions. Causing the reaction to move quicker to equilibrium.
|
|
|
True of False. Catalysts decreases the numerical value of the equilibrium constant?
|
False. the equilibrium constant depends on the thermodynamic properties of the reaction.
|
|
|
What are the characteristics of Equilibrium Constant (Keq)?
|
1) Solids and liquid are not included
2) Temperature is affects it 3) If Keq>1 = more products 4) if Keq<1 = more reactants 5) If Keq is close to 1 then reactants and products are close to equal. |
|
|
What changes the position of equilibrium and what changes the numerical value of equilibrium?
|
Temperature, Pressure, Volume, Reaction species concentration all change the position of equilibrium (shifting left or right)
ONLY Temperature can change the numerical value of the equilibrium constant. |
|
|
Paramagnetic molecules:
I. Have no unpaired electrons II. contain ferromagnetic domains III. are attracted into a magnetic field. |
III. ONly
Paramagnetic materials are those that are attracted into a magnetic field. Therefore we can immediately eliminate choices A, B, and D. All paramagnetic materials have at least one unpaired electron, so choice E can be removed. The only remaining answer is choice C. |
|
|
Which of the following is always true of an SN2 reaction involving a chiral substrate?
A. Choice of solvent is unimportant B. Absolute configuration is retained C. Absolute configuration is inverted D. The reaction rate is of second order overall E. Chloride is a better leaving group than bromide |
D. The reaction rate is second overall.
Choices B and C are incorrect be/c they can be either true or false depending on the identity of the species invloved. Relative configuration is inverted b/c the nucleophile attacks from the back, but absolute configuration depends on the relative priorities of the groups. |
|
|
Which of the following is always true of an SN2 reaction involving a chiral substrate?
A. Choice of solvent is unimportant B. Absolute configuration is retained C. Absolute configuration is inverted D. The reaction rate is of second order overall E. Chloride is a better leaving group than bromide |
D. The reaction rate is second overall.
Choices B and C are incorrect be/c they can be either true or false depending on the identity of the species invloved. Relative configuration is inverted b/c the nucleophile attacks from the back, but absolute configuration depends on the relative priorities of the groups. |
|
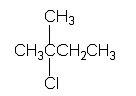
Will this compound, forming a Grignard product, be fast or slow?
|
slow
Formation of a Grignard product is favored when the resulting structure provides the greatest stabilization of the negative charge on the organic portion. - Electron-donating groups destabilize negative charges while electron-withdrawing groups (electronegative substituents) stabilize negative charges. The Grignard product places the negative charge on the carbon atom bonded to the halogen in the initial structure. Since alkyl groups are electron-donating, the compound in which the negative carbon is the most highly substituted will from the least stable Grignard product. |
|
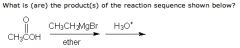
|
This is a "trick" question.
One normally expects the alkyl portion of the Grignard reagent to add to the carbonyl carbon. However, while Grignard reagents are strong nucleophiles, they are also strong bases. When the substrate is an acid (as in this case), the Grignard reagent strips off the substrate, forming the alkane. In the second step, reaction with the carboxylate salt with aqueous acid regenerates the carboxylic acid. |
|
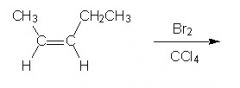
Would the product of this reaction be optically active or inactive?
|
Because the alkene is planar, the addition of Br+ (anti) can occur with equal probability to either side. Enantiomers form in equal proportions. A racemic mixture results = no optical activity. However, Remember chirality is a property of molecules. So just b/c chiral molecules are formed does not mean the molecule as whole will be optically active.
General Principle: we start with an optically inactive substrate and introduce only optically inactive reagents. It is impossible to generate optical activity along the way. |
|
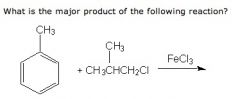
WHat is the product and what type of reaction is this?
|
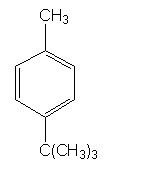
Friedel-Craft acylation
The reason why a t-butly groups is added para position is because it went rearrangement to form a more stable electrophile before it was attached to the ring. |
|
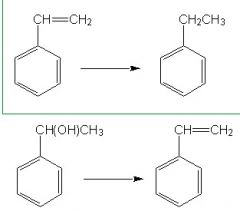
Between these two reaction which one is oxidized?
|
General Organic Chemistry oxidation rules:
OXIDATION - gaining C-O bonds, - gaining pi bonds (unsaturation) - losing hydrogen (dehydrogenation) REDUCTION: - losing C-O bonds - losing pi bonds (saturation) - gaining H (hydrogenation) In the top compound we see that a pi-bond has been lost, b/c it gained more hydrogens = reduction The bottom compound is neither reduction or oxidation it lost an OH but then gained pi bonds. |
|
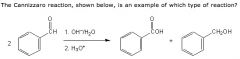
What is up with this reaction?
|
The reaction stars with two benzaldehyde molecules. One is oxidized into benzoic acid and the other is reduced into phenyl methanol. A reaction in which oxidation and reduction of the same species occur simultaneously is known as a disproportionation reaction.
|
|
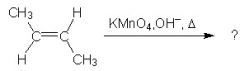
What is the product?
|
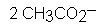
Hot permanganate solution is strongly basic oxidizing agent. It cleaves double bond of an alkene and forms 2 molar equivalents of carbonyl compounds.
In this case, the acid work-up is missing from the reaction scheme (which is why you don't see the two OH's attached). so the compounds remain in the conjugate base form. We end up with two molar equivalents of the acetate anion. |
|
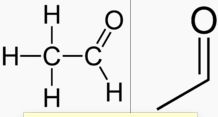
What is this?
|
acetaldehyde
|
|
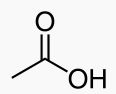
WHat is this?
|
acetic acid
|
|
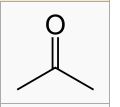
WHat is this?
|
acetone
|
|
|
THe purpose of sulfuric acid in the nitration of phenol is to...
|
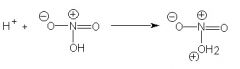
protonate the nitric acid molecule, thereby converting it into an electrophile
|
|

What is the result?
|
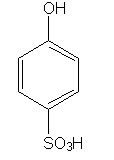
Fuming sulfuric acid consists of a mixture of sulfur trioxide and sulfuric acid. The reaction conditions given in the question are consistent with the addition of a sulfonate group to the benzene ring.
|
|
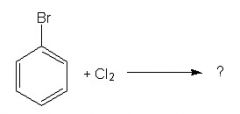
What is the product?
|
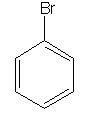
Trick:
Halogenation of the benzene ring requires the presence of a Lewis acid catalyst. To add Cl to the ring, we would need to use FeCl3 or AlCl3 in addition to chlorine. Since a Lewis acid catalyst is absent, no reaction occurs, and the product is the same as the reactant. |
|
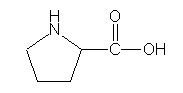
The five-membered imino ring of the amino acid proline prevents the alpha-amine nitrogen from participating in hydrogen bonding. Which of the following is true?
A. Proline cannot form peptide bonds. B. Proline cannot be part of a Beta-pleated sheet C. Proline cannot act as a Bronsted-Lowery base D. Proline is not amphoteric E. Proline does not contain sp2 hybridized atoms. |
B. Proline cannot be part of a Beta-pleated sheet.
Since secondary structures such as alpha-helices and Beta-pleated sheets arise from hydrogen-bonding interactions, proline will disrupt the Beta-sheet structure. |
|
|
What happens to gas solubility in a liquid when temperature is increased?
What happens to gas solubility of a liquid when the temperature is decreased? |
Temperatures increase = decrease in gas solubility in a liquid.
Temperature decreases = increase in gas solubility in a liquid. |

