![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
20 Cards in this Set
- Front
- Back
|
Contractile Apparatus in Skeletal Muscle
|
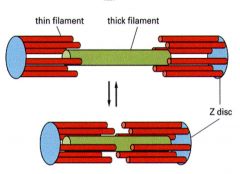
Thin and thick filaments, 3 ml wide with tapered ends
Every myosin is surrounded by 6 actin strands which is referred to as Interdigitated |
|
|
Components of myosin
|
Four Light Chains, Two Heavy Chain, Two globular heads, Rod Domain (helix) MAKING UP ABOUT 35% of Muscle Protein
One thick filament will have 600 head groups which pull the thin filament to the M line |
|
|
Function of Myosin
|
Myosin heads hydrolyze atp for mechanical movement and they self assemble at the tail ends
Myosin heads arranged at 30 degrees rotational symettry with thin filaments surrounding them at 60degrees |
|
|
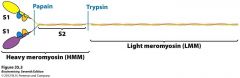
Myosin Fragments
|
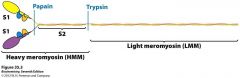
Proteases can cleave myosin at certain spots: papain and typsin make S1 and S2 fragments
|
|
|
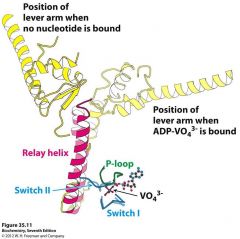
S1 Fragment and Light chain
|
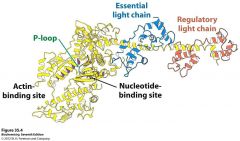
Has two light chains, p loop, actin binding site, and nucleotide binding site exhibiting a p loop atpase domain
Light chains act like calmodulin (smooth muscle) and bind the alpha helix by wrapping around it. This fragment also has different conformation depending on what is attached to it which allows the powerstroke to occur based on ATP hydrolysis |
|
|
Actin: componenets
|

Actin has a positive barbed end anchored to the Z disks and a negative pointy end which goes towards the M line.
The polarity is in such a way that actin heads will not bind with each other during a muscle contraction Has nucleotide binding sites which allow for interaction: these interactions (and hydrogen binding) allow for bonding |
|
|
Actin Synthesis and Formation
|
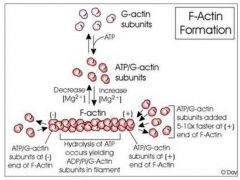
G Actin assembles into F actin (filamentous) in a double helical arrangement.
Each G actin interacts with four neighbors and the growing/final filament has directionality ATP binds to free actin first and the mature filament has ADP bound to monomeric G Actin Units |
|
|
Tropomodulin, Capz, Nebulin: what do they do
|
Tropomodulin caps the minus end to prevent more polymerization of F actin
CapZ (beta actinin) associated with alpha actinin to cap the plus end (z disk end) Nebulin acts as a ruler and template for actin polymerization |
|
|
Troponin T, I, and C on the thin filament
|
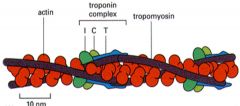
Troponin T - binds tropomyosin and positions the complex on the filament
Troponin I - binds actin and inhibits myosin binding Troponin C - binds calcium and relieves inhibition |
|
|
Tropomyosin and troponin complex
|
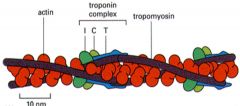
Tropomyosin has a coiled structure, wrapped and attached to the groove of the actin molecule.
The troponin complex is periodic and sits at certain parts The heart has isoforms of the troponin complex and during a heart attack, due to the change in conformation of cells and permeability, will leak small molecules first: therefore troponin in the blood is indicative of a heart attack |
|
|
M protein: Myomesin
Alpha Actinin Titin |
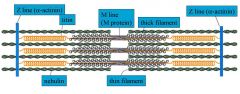
Myomesin helps provide arrangement at the M line
Alpha Actinin helps maintain architecture of the z disk and works with Beta Actinin to cap the z(plus) end of Actin Titin is the biggest protein: acts as a molecular ruler, useful for muscle development, helps balance forces across a sarcomere, provides passive elasticity and muscle signaling. Helps the muscle pop back to the ready state |
|
|
Dystrophin
|
Dystrophin is the biggest gene on planet
Dystrophin gene codes for the 427kDa protein, which is a rod-like protein that connects the cytoskeleton to basal lamina and stabilizes the membranes and participates in calcium handling. C terminal Variability occurs from alternative exon splicing and N terminal variability occurs from alternative transcription initiation sites |
|
|
Duchenne Muscular Dystrophy
Becker Muscular Dystrophy |
DMD- no detectable dystrophins at all
BMD- dystrophins of various sizes Mostly caused by exon deletion and less commonly exon duplication |
|
|
Desmin and Synemin
|
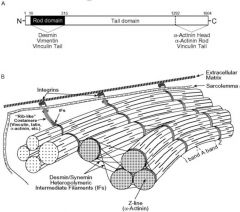
Synemin and Desmin both work together to help anchor the myofibril at the zdisk
|
|
|
Molecular Basis of Contraction (the simple version)
|
Tropomyosin blocks myosin binding site on Actin
When calcium is introduced, the tropomyosin will come off of the myosin binding site and now the myosin binding site will be exposed. This allows myosin to bind to actin |
|
|
Smooth Muscle Contraction
|
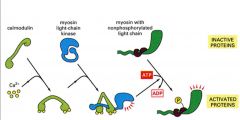
1. Calmodulin binds with Calcium
2. The calmodulin/calcium complex will bind with myosin light chain kinase and activate the myosin light chain kinase 3. This activated complex will phosphorylate Myosin and this phosphorylation will initiate the contraction and activate myosin ATPase 4. |
|
|
Myosin Power Stroke
|
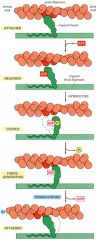
1. Atp binds to myosin and this causes myosin to dissociate from actin
2. Myosin will hydrolyze ATP, changing the conformation again and bringing it to a cocked position 3. When the phosphate from the hydrolyzed ATP is dissociated, the myosin head will reassociate with actin at a new position 4. when the final ADP is dissociated, a power stroke will occur |
|
|
Adenylate Kinase Reaction: roll in AMP mediated muscle glycogenolysis and glycolysis
|
As muscle contracts, ATP is converted to ADP and P. in the Adenylate kinase reaction, two ADP molecules will react to form ATP and AMP. ATP will be used for contraction and AMP will activate glycogenolysis and glycolysis
|
|
|
AMP and phosphate mediated muscle glygogenolysis
|
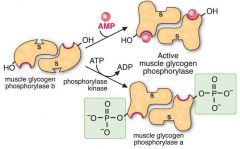
Muscle Glygogen Phosphorylase, made up of two identical subunits is activated by AMP.
AMP will bind to an allosteric site, causing a change in the active site which will increase enzyme activity Also, phospohorylation of a specific serine residue can cause a change to the active site which will increase enzyme activity as well (done via inorganic phosphate Vasu thinks, but will double check/probably isn't important) |
|
|
AMP mediated muscle glycolysis
|
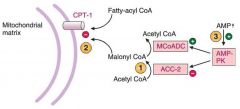
Acetyl CoA carboxylase 2 (ACC-2) will convert AcetylCOA to malonylCOA which inhibits CPT1 preventing Fatty AcylCOA from entering.
AMP will come and activate AMP-PK which will phosphorylate and INACTIVATE ACC-2 and phosphorylate (this will prevent more MCoA from forming) and ACTIVATE malonylCOA decarboxylase (MCoADC) (which will help convert MCoA into AcetylCoA) This will ALL make CPT1 functional and allow acylCoA to enter into the mitochondria |

