![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
33 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Dalton |
Measured the masses of reactants and products. Proposed atoms. Atoms are never created or destroyed. |
1803 |
|
|
Thompson |
Deflected particles is cathode ray tube. Atoms have + & - charges. Negative charge = electrons. Plum Pudding Atom Model. |
1897 |
|
|
Millikan |
Used oil drops in an electric field of known strength to find charge-to - masses ratio of electrons. |
Random guy that no one on YouTube talks about |
|
|
Rutherford |
Fired alpha particles through gold foil. Measured the scatter patterns and found that the atoms were deflected by the foil. The mass of the atom is concentrated at the center and the rest of it is mostly empty space. |
1909 |
|
|
Planck |
Electromagnetic energy is quantization (composed of bundles).
E=hv OR E=hc/wavelength |
|
|
|
Bohr |
Electrons have fixed radius.
Electrons with higher energy are further from center.
Bohr model (planetary model) only accurate for atoms with 1 electron.
Electrons with more energy give off more electromagnetic radiation. Quantum leaps: electrons jump energy levels. |
1913 |
|
|
de Broglie |
Identified wave characteristics of matter by combining E=mc^2 and E=hv. All particles have a wave nature. |
|
|
|
Heisenberg |
It is impossible to know both the position and momentum of the electron at the same time. We need a wave model to understand electron behavior. |
|
|
|
Schrodinger |
Attributed a wave function to electrons. The wave function describes where an electron might exist. High probability areas are called orbitals. This is called the Electron Cloud Model |
|
|
|
Orbital Types |
s, p, d, f |
|
|
|
Atomic Mass |
Mass of protons + mass of neutrons |

|
|
|
Atomic Number |

Total nuclear charge
# of protons |

|
|
|
Isotopes |
Atoms with same # of protons but different # of nuetrons. Carbon-12 and Carbon-14 |
|
|
|
Average Mass Number du(atomic weight) (molar mass) |

Takes into account the frequencies of different isotopes Molar Mass: the mass in grams of 1 mole of atoms. 6.02 × 10^23 atoms = 1 mole Atomic weight is closest in value to the most common isotope. |
|
|
|
Electrons |
Three ways to locate electrons. (Quantum numbers, orbital notation, electron configurations) |
|
|
|
Pauli Exclusion Principle |
No 2 electrons can occupy the same energy level or have the same set of 4 quantum numbers. |
|
|
|
Quantum Numbers |
Used to locate electrons. There are four of them. Distance from nucleus Type of orbital filled Orientation of the orbital Spin direction of electron |
|
|
|
Orbital Notation |
Identifies location and parallel spin of electron. |
|
|
|
Electron Configurations |
Identify the # of electrons in each type of orbital at each energy level. |
|
|
|
Aufbau Principle |
Electrons exist first at the lowest energy level until something g excites them. |
|
|
|
Hund's Rule |
Electrons enter orbitals of equal energy singly with the same spin before they become paired. |
|
|
|
Principal Quantum Number (n) |
Represents the shell / energy levels. Aka: distance from the nucleus. Possible values of 1-7 |
|
|
|
Angular Momentum Quantum Number (l) |
Represents the subshell or orbital type. When n=1, l=0 (s orbital) When n=2, l=0 or 1 (s, p) When n=3, l= 0,1, or 2 (s, p, d) When n=4, l= 0, 1, 2, or 3 (s, p, d, f) |
|
|
|
Magnetic Quantum Number (m1) |
Represents the orbital position.
When l=0, m1=0 (1 s orbital) When l=1, m1= -1, 0, 1 (3 p orbitals) When l=2, m1= -2, -1, 0, 1, 2 (5 d orbitals) When l=3, m1= -3, -2, -1, 0, 1, 2, 3 (7 f orbitals) Orbital with the most negative # is filled first. |
|
|
|
Magnetic Spin Quantum Number |
Each orbital can contain 2 elections. 1 electron has a positive spin, 1 has a negative spin. The first electron always has a positive spin. |
|
|
|
Diamagnetic Elements |
Have a paired electron in each orbital. "Stable" All subshells are filled and are not affected by magnetic fields. |
|
|
|
Paramagnetic Elements |
Have an unpaired electron in at least one orbital. The unpaired electron creates a magnetic field that responds to external magnetic fields. |
|
|
|
Atomic Radii |
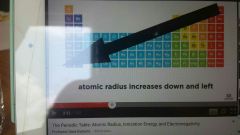
Size of the atom |
|
|
|
Ionization Energy |

Energy required to remove an electron from the atom |
|
|
|
Electron Affinity |
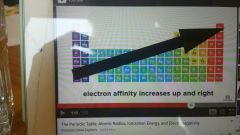
How much an atom wants to gain an electron
|
|
|
|
Electronegativity |
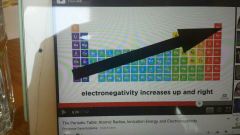
Ability of an atom to hold tightly to an atom |
|
|
|
Ionic Attractions |
Combination of metal and non-metal Difference in electronegativity greater than or equal to 1.7 Electron leaves the less electronegativity atom and creates a positive charge. When it joins the other atom, a negative charge is formed. The two atoms are now attracted to eachother. Bond, ionic bond. Electrons taken, not shared. |
|
|
|
Covalent Bonds |
Occur between non-metals
Electromagnetic difference between 0 and 1.7.
Electrons are shared # of possible covalent Bonds = 8 - group # of the element. |
|

