![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
25 Cards in this Set
- Front
- Back
|
Ionic Bonds
|

metal + nonmetal
- relatively strong - electrons transferred from one to another - large array example: table salt -cations and anions form and are held together electrostatically |
|
|
Properties of Ionic Compounds
|
1) Crystalline solids (made of ions)
2) High melting and boiling points 3) Conduct electricity when melted or dissolved in polar liquid 4) Many soluble in water but not in nonpolar liquid - water molecules attract ions to dissolve compound 5) ions held in place by lattice |
|
|
Covalent Bonds
|
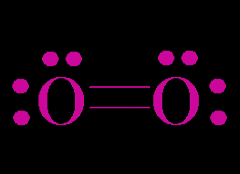
nonmetal + nonmetal
- electrons shared and localized - travel back and forth example: water |
|
|
Properties of Covalent Compounds
|
1) Gases, liquids, or solids (made of molecules)
2) Low melting and boiling points 3) Poor electrical conductors in all phases 4) Many soluble in nonpolar liquids but not in water |
|
|
Metallic Bonds
|
metal + metal
- electrons through entire crystal lattice - electron pool example: sodium |
|
|
Properties of Metallic Compounds
|
1) High melting and boiling points
2) conduct electricity and heat 3) hard and dense 4) malleable (can be hammered into shapes) 5) ductile (can be made into wires) |
|
|
Why do chemical bonds form?
|
- they lower potential energy between the charged particles in the constituent atoms
- potential energy of bonded atoms is less than that of separate atoms |
|
|
What are the guidelines for drawing a Lewis Structure for an atom or molecule?
|
1) find total # of valence electrons
2) draw backbone structure 3) ensure each atom has its duet or octet, keep in mind exceptions (row 3, 2, or 1 with empty d orbitals). |
|
|
Lewis Bonding Theory
|
- atoms bond to obtain noble gas configuration if possible
- cations have Lewis symbols without valence electrons because they gave them away - anions have Lewis symbols with 8 valence electrons *brackets and charge around symbol - structures used as predictors of how atoms combine to form compounds and molecules |
|
|
Lattice Energy
|
- the energy required to separate a mole of an ionic solid into gaseous ions (or bond strength)
- released as heat - gaseous cations reacting with gaseous anions to form solid compound - electron affinity and ionization energy are also involved |
|
|
Trends in Lattice Energy
|
- size: shorter distance between ions means higher stability
- charge: larger charges, more stable - Coulomb's Law: multiply charges and divide by distance |
|
|
Born-Haber Cycle Definition and Steps
|
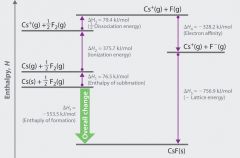
to calculate lattice energy
|
|
|
Lattice Energy and Physical Properties
|
- the higher the lattice energy, the less soluble a compound is in water (harder to pull apart)
- higher lattice energies associated with higher charges on the ions - the higher the lattice energy, higher the melting point - halides: melting points get lower down the column |
|
|
Electronegativity and Trends
|
- ability of an atom to attract electrons to itself resulting in ionic and polar covalent bonds
- F is most electronegative (x=4.0) - O is second most electronegative (x=3.5) - increases across table - decreases down table - Cs is most electropositive (x=0.7) (least electronegative) |
|
|
Bond Polarity Classification
|

- dipole moment will point towards more electronegative end with a plus on more electropositive end
|
|
|
Percent Ionic Character
|
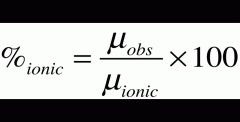
- 6.2D is the ideal total charge-transferred dipole moment (M ionic)
|
|
|
Resonance Structures
|
- resonance structures differ in the position of multiple bonds and non bonding electron. The placement of atoms and single bonds always stays the same.
- only electrons move, not nuclei - only pi electrons, single unpaired electrons, and lone pair electrons can move - total number of electrons in the molecule do not change and neither do the number of paired and unpaired electrons *favored: 1) negative formal charge on most electronegative atom 2) fewer and smaller formal charges |
|
|
Formal Charge
|
- when atoms end up with more or fewer electrons than they brought
- sum of formal charges in a molecule = 0 - in an ion, total = the charge Steps 1) for each atom, count the electrons in lone pairs and half the electrons it shares with other atoms. 2) subtract that from the number of valence electrons for that atom: the difference is its formal charge |
|
|
Bond Energies
|
- breaking is endothermic, takes energy (+)
- forming is exothermic, releases energy (-) - ^Hrxn reaction energy |
|
|
Bond Lengths
|
- distance between nuclei of bonded atoms
- avg bond length: b/c actual bond length depends on other atoms around the bond - single bods are longest and weakest - triple bonds are shortest and strongest |
|
|
VSEPR
|
- valence shell electron pair repulsion
* depends on how many electron groups are bonding and how many are lone pairs - electron groups repel one another through Coulombic forces - repulsions between electron groups on interior toms determine geometry of a molecule - preffered geometry: in which electron groups have max separation |
|
|
Molecular and Electron Geometry
|
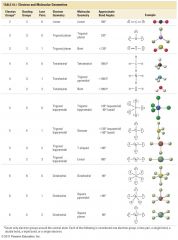
|
|
|
Bond Polarity
|
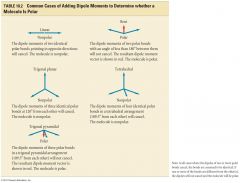
- dipole moments are vectors and add according to vector algebra (can cancel or reinforce each other)
- vectors have both magnitude and directionn |
|
|
Predicting Polarity
|
1) draw lewis structure
2) determine if bonds are polar (atoms have different electronegativities) 3) determine whether polar bonds add together to give a net dipole moment |
|
|
Polarity and Solubility
|
- polar attracts polar
- polar dissolves well in water (polar) and in ionic compounds - some molecule are partially polar and nonpolar |

