![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
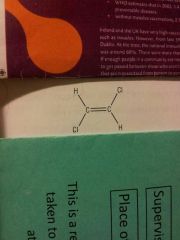
|
E isomer Trans isomer-Groups on opposite sides 1, 2-dichloroethene |
|
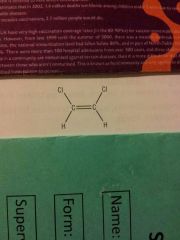
|
Z isomer Cis isomer- groups on the same side 1, 2 dichloroethene |
|
|
What is homolytic fission? |

|
|
|
What is a radical? |
A species with an unpaired electron |
|
|
What is heterolytic fission? |

|
|
|
What is a nucleophile? |
An atom that is attracted to an electron deficient centre or atom, where it donates a pair of electrons to form a new covalent bond |
|
|
What is an electrophile? |
An atom that is attracted to an electron rich centre or atom, where it forms a new covalent bond |
|
|
What is an addition reaction? |
A reaction where a reactant is added to an unsaturated molecule to make it saturated |
|
|
Combustion of an alkane produces...? |
CO2 and water BALANCE THE EQUATION! |
|
|
Incomplete Combustion of an alkane produces...? |
CO and Water BALANCE THE EQUATION! |
|
|
What is Cracking? |

|
|
|
What is a catalyst? |

|

