![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
71 Cards in this Set
- Front
- Back
|
Why is lab safety important?
|
so you know what to do in case of an emergency
|
|
|
What are a few of the safety rules?
|
Listen to your teacher. Be alert. Tie up hair. Know where the nearest fire alarm is. Wear protective clothing, waft the fumes, do not chew gum, eat or drink. Be careful. Clean up.
|
|
|
Safety rules?
|
Never leave an open flame unattended. Never use broken or chipped glassware. Never engage in horseplay.
|
|
|
What is matter?
|
Anything that has mass and volume.
|
|
|
What is mass?
|
Mass is the amount of matter in a substance or object.
|
|
|
What is volume?
|
Volume is the amount of space a substance or object occupies.
|
|
|
What is a chemical change?
|
A change in matter that occurs when substances combine to form new substances. New chemical bonds are formed, while other chemical bonds are broken.
|
|
|
What is a physical change?
|
A substance changes form but not chemical composition. No new substances are formed.
|
|
|
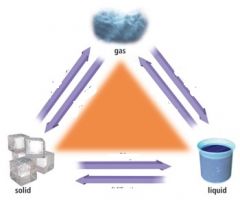
What are the three states of matter?
|
Solid, liquid, gas.
|
|
|
Does a solid have a definite shape and a definite volume?
|
Yes to both
|
|
|
Does a liquid have a definite shape and a definite volume?
|
No to definite shape. Yes to definite volume.
|
|
|
Does a gas have a definite shape and a definite volume?
|
No to both.
|
|
|
Describe the 4 points in the
Particle Model of Matter: & in the Kinetic Molecular Theory: |
1. All matter is made up of tiny particles.
2. There are empty spaces between the particles. In gases, the spaces are farther apart than in liquids and solids. 3. Particles of matter are always moving. 4. The more energy particles have, the faster they can move and the farther apart they get. |
|
|
What does kinetic mean?
|
The energy of motion.
|
|
|
Why do the particles of solids, liquids, and gases all have kinetic energy?
|
Because particles are always moving.
|
|
|
How do particles in a solid move?
|
Particles are very close to one another, fixed in position, and vibrate.
|
|
|
How do particles in a liquid move?
|
All particles are still close, but now have enough space to slide past one another.
|
|
|
How do particles in a gas move?
|
All particles are highly energetic and move freely to spread out in their container. Further heating gives particles even more kinetic energy, making the gas spread out faster and farther.
|
|
|
Which state has the most kinetic energy? Why?
|
Gases because they have the most space, which means the most movement, which equals kinetic energy.
|
|
|
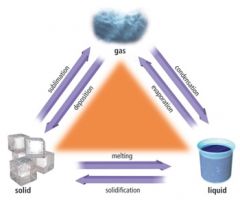
As more energy and heat is added to particles, what happens?
|
Kinetic energy increases. Movement increases. Space increases.
|
|
|
|
|
|
________ is the change of state from a solid to a liquid.
|
Melting.
|
|
|
The temperature at which this takes place is called the ___________ and is __________ degrees Celsius for water.
|
melting point
0 |
|
|
________ is the change of state from a liquid to a gas.
|
Boiling
|
|
|
The temperature at which this takes place is called the ______________ and is ________ degrees Celsius for water.
|
boiling point
100 |
|
|
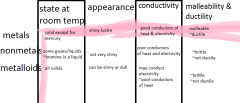
Physical properties of matter are ___________________________________.
|
characteristics of matter that can be observed or measured.
|
|
|
Qualitative properties can be _____________ but not _________________. Examples of these properties are:
|
described
measured state, color, malleability, ductility |
|
|
Quantitative properties can be ____ _______. Examples are:
|
measured numerically
solubility, conductivity, density, viscosity |
|
|
What is a pure substance?
|
A substance made up of only one kind of particle.
|
|
|
What are the two kinds of pure substances?
|
elements and compounds
|
|
|
Two examples of elements:
|
Oxygen and gold
|
|
|
The two types of compounds in chapter 3:
|
ionic compounds
covalent compounds |
|
|
Location, charge:
proton neutron electron |
nucleus, positive
nucleus, no charge, shells, negative |
|
|
What are the subatomic particles that have mass?
|
Protons and neutrons.
|
|
|
Which subatomic particles make up most of the volume of the atom?
|
Electrons.
|
|
|
What charge does the nucleus have?
|
Positive.
|
|
|
Atoms are neutral, even though protons and electrons have charge. How is this possible?
|
Protons and electrons balance out the charge.
|
|
|
If an atom loses electrons, it becomes a _______ ion.
|
positive
|
|
|
If an atom gains electrons, it becomes a _________ ion.
|
negative
|
|
|
Each element is made up of:
|
only one kind of atom.
|
|
|
What is an element?
|
A pure substance that cannot be broken down or separated into simpler substances.
|
|
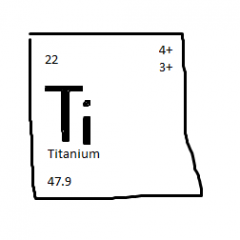
top right:
bottom left: top left: middle letters: |
ionic charges
atomic mass atomic number chemical symbol |
|
|
Atomic number is the number of:
|
PROTONS in the atom of an element
|
|
|
What is atomic mass?
|
Mass of an average atom.
|
|
|
What defines the element?
|
Atomic number.
|
|
|
What is the ionic charge?
|
Electric charge that forms when an atom gains or loses electrons.
|
|
|
When an atom gains a charge, it is then called a ____.
|
Ion
|
|
|
MEMORIZE.
|
|
|
Far left column, then next column.
Column beside far right column, far right column |
Alkali Metals
Alkaline Earth Metals Halogens Noble Gases |
|
|
What is a period on the periodic table? How many are there?
|
A horizontal row. Seven.
|
|
|
What is a family on the periodic table?
|
Vertical columns are families. There are four to know.
|
|
|
Alkali Metals. Group 1.
|
Shiny, silvery, soft metals. Forms water soluble compounds. Highly reactive.
|
|
|
Alkaline Earth Metals. Group 2
|
Shiny, silvery metals. Forms water insoluable compounds. Less reactive than alkali, but will burn in air if heated.
|
|
|
Halogens. group 17
|
Highly reactive, react readily with alkali metals
|
|
|
Noble Gases. Group 18
|
colourless, odorless, gases. Most stable and nonreactive elements in the periodic table. Do not form compounds easily
|
|
|
Electron amount in the shells:
|
2, 8, 8, 18, 18, 32
|
|
|
Electrons in the outer shell are called:
|
valence electrons
|
|
|
The shell that contains outer electrons is called the:
|
valence shell
|
|
|
Most elements in the same family have the same:
|
number of valence electrons.
|
|
|
The period number =
|
the number of shells an atom has.
|
|
|
Valence electrons increase by 1 in the:
|
same period.
|
|
|
Why are noble gases stable and unreactive?
|
Full electrons shells. they have a stable octet
|
|
|
Metals _____ electrons and become _______.
|
lose
cations |
|
|
Nonmetals ______ electrons and become ________
|
anions
|
|
|
What is a compound?
|
A compound is a pure substance made up of two or more elements that are chemically combined.
|
|
|
What are chemical bonds?
|
They are links that hold atoms together. Help form compounds.
|
|
|
What is a covalent compounds?
|
Compounds in which two atoms share a pair of electrons.
|
|
|
How do covalent compounds form?
|
By sharing electrons.
|
|
|
What is an ionic compound?
|
Compounds in which oppositely charged ions come together because of mutual attraction.
|
|
|
In ionic compounds, atoms ___________ electrons to form ions.
|
gain or lose
|
|
|
What is a polyatomic ion?
|
A molecular ion composed of more than one type of atom joined by covalent bonds. Prefix "poly" means many.
|

