![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
44 Cards in this Set
- Front
- Back
|
Clausius–Clapeyron relation (Vapor Pressure)
|
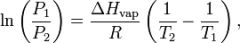
|
|
|
The cubic lattice types in which crystals have been known to crystallize are
|
simple cubic
face centered cubic body centered cubic |
|
|
number of atoms in a face centered cubic unit cell
|
4
|
|
|
number of atoms in a body centered cubic unit cell
|
2
|
|
|
number of atoms in a simple cubic unit cell
|
1
|
|
|
Viscosity
|
Resistance of liquid to flow
|
|
|
Surface tension
|
The energy required to expand a liquid surface
|
|
|
Volatility (of a liquid)
|
The tendency of a liquid to vaporize (increases as temperature increases)
|
|
|
Vapor Pressure
|
A state of dynamic equilibrium in which molecules enter and leave the liquid at equal rates.
OR The pressure of a vapor in equilibrium with its non-vapor phases |
|
|
Boiling
|
When the vapor pressure equals the atmospheric pressure
|
|
|
Relative Humidity Formula
|
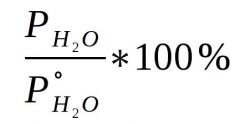
Vapor Pressure of atmosphere divided by equilibrium partial pressure of water vapor at the relevant temperature
|
|
|
Relative Humidity
|
Vapor pressure of of water in the atmosphere
|
|
|
Vaporization
|
liquid -> vapor
|
|
|
Condensation
|
vapor -> liquid
|
|
|
ΔHºvap or ΔvH - Definition
|
Enthalpy of vaporization - Energy required to transform a given quantity of a substance into a gas.
Equals -ΔHºcondensation |
|
|
ΔHºsublimation - Definition
|
Enthalpy of sublimation - Energy required to transform a given quantity of a substance into a gas.
Equals -ΔHºdeposition |
|
|
ΔHºfusion - Definition
|
Enthalpy of fusion - Energy required to transform a given quantity of a substance into a liquid.
Equals -ΔHºcrystallization |
|
|
Fusion
|
Solid -> liquid
|
|
|
Crystallization
|
liquid -> solid
|
|
|
Sublimation
|
solid -> gas
|
|
|
Deposition
|
gas -> solid
|
|
|
Triple point
|
The point in a phase diagram at which all 3 phases of matter are at equilibrium
|
|
|
Phase Diagram
|
chart used to show conditions at which thermodynamically-distinct phases can occur at equilibrium. Every pure substance that exists in all three phases has a distinct phase diagram
|
|
|
Types of solids
|
Ionic
Metallic Molecular Network Amorphous |
|
|
Ionic solid - Structural Units
|
positive & negative ions; no discrete molecules
|
|
|
Ionic sold - Forces holding units together
|
Ionic bonding
|
|
|
Ionic solid - Typical properties
|
Hard, brittle; high melting point, poor electrical conductor as solid, good as molten liquid; often water soluble
|
|
|
Metallic solid - Structural Units
|
Metal atoms
|
|
|
Metallic sold - Forces holding units together
|
Metallic bonding
|
|
|
Metallic solid - Typical properties
|
Malleable; ductile; good conductor
|
|
|
Molecular solid - Structural Units
|
Molecules with covalent bonds
|
|
|
Molecular sold - Forces holding units together
|
London Forces, dipole-dipole forces & hydrogen bonds
|
|
|
Molecular solid - Typical properties
|
low melting & boiling point; soft; poor electrical conductor
|
|
|
Network solid - Structural Units
|
Atoms held in infinite one-, two- or three-dimensional network
|
|
|
Network sold - Forces holding units together
|
Covalent bonds
|
|
|
Network solid - Typical properties
|
hardness and melting points decrease with # of bonds in network; poor electrical conductor
|
|
|
Amorphous solid - Structural Units
|
Covalently bonded networks of atoms or collections of large molecules
|
|
|
Amorphous sold - Forces holding units together
|
Covalent bonds
|
|
|
Amorphous solid - Typical properties
|
Noncrystalline; wide temperature range for melting; poor electrical conductor
|
|
|
Crystal lattice
|
The orderly, repeating arrangement of ions, molecules or atoms that shows the position of each particle
|
|
|
Unit cell
|
A small part of a lattice that, when repeated, reproduces the entire crystal structure
|
|
|
Simple cubic edge - formula
|
edge = 2 * atomic radius
|
|
|
face-centered cubic edge - formula
|
edge = 4 * atomic radius / sqrt(2)
|
|
|
face-centered cubic edge - formula
|
edge = 4 * atomic radius / sqrt(3)
|

