![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
11 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
4 properties of gases |
No definite volume Diffusion Fluidity Low density |
|
|
|
Ideal gases |
Molecules have mass but no volume Do not attract any other molecules |
|
|
|
Avogadro's Principle |
At equal temperatures and pressures equal volumes of gases contain equal number of molecules |
|
|
|
5 gas values |
101.325kpa 273K 22.414 dm^3/mol 8.13 (dm^3)(kpa)/(mol)(k) 1.29 g/dm^3 |
STP Molar volume Universal gas constant Density of air |
|
|
Boyle's law |
(V1)(P1)=(V2)(P2) |
|
|
|
Charles' Law |
V1/T1=V2/T2 |
Change to Celsius to Kelvin |
|
|
Combined gas law |
(V1)(P1)/T1=(V2)(P2)/T2 |
Change Celsius to Kelvin Subtract kpa for pressure |
|
|
Ideal gas equation |
(P)(V)=(n)(R)(T) |
|
|
|
Dalton's law |
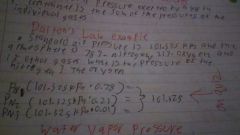
Pn1=(101.325kpa)(X%)=101.325 |
|
|
|
Density/specific gravity |
D=M/V Sp.Gr.=DSub/DAir |
|
|
|
Stoichiometry |
Mole/coefficient Mass/(coefficient)(molar mass) Volume/(coefficient)(molar volume) |
|

