![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
|
2.18 describe the laboratory preparation of oxygen from hydrogen peroxide, using manganese(IV) oxide as a catalyst
|
Hydrogen peroxide is put in a conical flask with manganese(IV) oxide as a catalyst. Plus there could be some water to dilute the hydrogen peroxide.
Hydrogen peroxide > water + oxygen 2H2O2 > 2H2O + O2 You can then collect oxygen by downwards displacement method. |
|
|
3.5 describe the substitution reaction of methane with bromine to form bromomethane in the presence of UV light.
|
In UV light bromine and methane will form bromomethane:
CH4 + Br2CH3Br + HBr What has happened in this reaction is a bromine has taken the place of a hydrogen (substitution). |
|
|
3.12 describe the dehydration of ethanol to ethene, using aluminium oxide.
|
C2H5OH > C2H4 + H2O
ethanol > ethene + water aluminium oxide is the catalyst for this reaction. |
|
|
3.10 describe the manufacture of ethanol by the fermentation of sugars, for example glucose, at a temperature of about 30°C
|
Ethanol can be made by the anaerobic respiration of microorganisms.
glucose > ethanol + carbon dioxide This happens at 30 degrees. |
|
|
3.9 describe the manufacture of ethanol by passing ethene and steam over a phosphoric acid catalyst at a temperature of about 300°C and a pressure of about 60–70 atm
|
C2H4 (ethene) + H2O (steam) > C2H5OH (ethanol)
This reaction takes place at a high pressure (60-70 atmospheres) and a high temperature (300) to make the reaction happen very quickly. Phosphoric acid also speeds up the reaction as it is a catalyst. |
|
|
3.8 describe the addition reaction of alkenes with bromine, including the decolourising of bromine water as a test for alkenes
|
An alkene will make its double bond into a single bond, to bond to two bromines. Bromine is added to the molecule. The product made is colourless. When alkenes are put in bromine water it turns from brown to colourless (a good way of testing for alkenes.)For example:
C2H4(g) + Br2 (aq) → C2H4Br2 (aq) |
|
|
5.14 describe how long-chain alkanes are converted to alkenes and shorter-chain alkanes by catalytic cracking, using silica or alumina as the catalyst and a temperature in the range of 600–700C.
|
Long chain hydrocarbons are passed over a hot catalyst (silica or alumina at 600-700 degrees) this causes them to break down into smaller molecules. As some atoms are lost from molecules, they become unsaturated and can therefore from a double bond. This is how you get alkenes from the process as well as shorter chain alkanes.
ttp://www.bbc.co.uk/schools/gcsebitesize/science/aqa_pre_2011/oils/polymersrev1.shtml |
|
|
5.23 describe the manufacture of ammonia by the Haber process, including the essential conditions
|
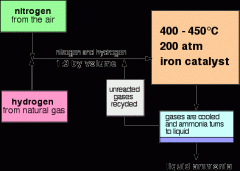
i) a temperature of about 450°Cii) a pressure of about 200 atmospheresiii) an iron catalyst
|
|
|
5.27 describe the manufacture of sulfuric acid by the contact process, including the essential conditions
|
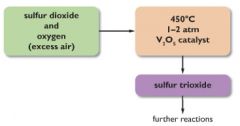
(stage 1) S + 2O > SO2
(stage 2) SO2 + O > SO3 (stage 3) SO3 + H2O > H2SO4 |

