![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
24 Cards in this Set
- Front
- Back
|
What is the trend for ionization energy? |
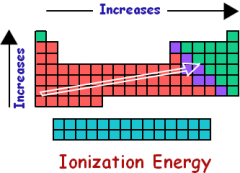
Ionization energy increases from the bottom left to the top right of the periodic table. |
|
|
What is the trend for electronegativity? |
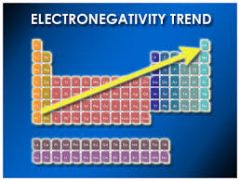
Electronegativity increases from the bottom left to the top right of the periodic table. |
|
|
What is the trend for electron affinity |
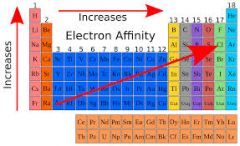
Electron affinity increases from the bottom left tot the top right of the periodic table. |
|
|
What is the definition of ionization energy? |
Ionization energy is the amount of energy that is needed to remove an electron from a a gaseous atom(formation of a cation). |
|
|
What is the definition of atomic radius? |
The distance between the center and most outer electrons of an atom. |
|
|
What is the trend for atomic radius? |
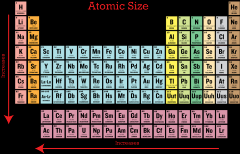
the atomic radii increases from the top right to the bottom left of the periodic table. |
|
|
What is the definition of electronegativity? |
The relative strength of attraction of an atom's nucleus to a bonding pair of electrons. |
|
|
What is the definition of electron affinity? |
The energy released when an electron is added to a gaseous atom(formation of anions). |
|
|
What is the formula to calculate the average atomic mass of an element? |
M=mass x=element being calculated A=abundance Mxavg = (ax1 * mx1) + ( ax2 * mx2) + ... all answers are stated in atomic mass units. |
|
|
What is significant digits? |
all digits included in a stated value, except leading zeros. ex: 0.026 has 3 significant digits 0.045600 has 5 significant digits
Always use the number with the smallest amount of significant digits as the same number of significant digits in your answer |
|
|
How are covalent compounds formed and what are they? |
Covalent compounds are compounds with two or more non metal elements. They are formed when bonding atoms share electrons to become stable. |
|
|
What is an example of a lewis structure. |
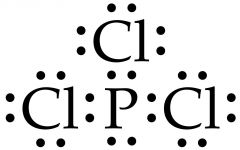
Phosphorus is sharing 3 electrons, one with each chlorine. |
|
|
What is an example of a structural formula. |

Strucural formula uses lines to illustrate the bonding pair of electrons and non bonding electrons remain as dots. |
|
|
What is the electronegativity difference and how does it affect the drawing of a lewis structure/structural formula. |
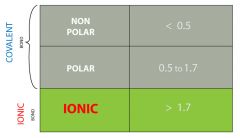
By subtracting the smaller relative electronegativity from the larger relative electronegativity of a pair of bonding atoms the strength and type of bond can be determined. if the difference is 0.0 it is a pure covalent bond. |
|
|
How do you know whether a reaction will occur during a single displacement reaction? |
an activity series is used to show which metals are more active than others. If the metal has a higher activity it will displace the metal ion dissolved in solution from the solution. if the metal has a lower activity then the metal in the compound no reaction will occur. their is a similar series for nonmetals. |
|
|
What is used to decide the products of a double displacement reaction? |
the solubility rules are used to determine whether the products will be solids, liquids or gasses or dissolved in water(aqueous). |
|
|
What is a mole? |
a mole(mol) is a unit for a quantity of particles. These particles can be individual atoms, molecules, or even electrons. the number of particles that make up a mole was settled on 6.022 * 10^23. |
|
|
What is the law of definite proportions?
|
the law of definite proportion states that the composition of a specific compound was determined to be constant. the reactant to product ratio was equal.
|
|
|
What is the formula used to calculate the percent composition of a compound? |
percent composition of ab =(molar mass of a/molar mass of ab) * 100% |
|
|
What is stoichiometry? |
Stoichiometry is the science of determining relative proportions between chemicals in a reaction. This is done using the molecule to mole relationship. |
|
|
What is percent yield? |
Percent yield is the ratio in the form of a percent of the actual yield compared to the theoretical yield. |
|
|
What is the actual yield? |
the actual yield is the actual mass of the compound that has been produced. |
|
|
What is the theoretical yield? |
the theoretical yield is the expected mass of the compound formed that could be produced if the reaction occurred perfectly(this never happens). |
|
|
What is the formula used to calculate percent yield? |
percent yield = (actual yield/theoretical yield) * 100% |

