![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
13 Cards in this Set
- Front
- Back
|
What is precipitation reaction? |
A reaction which is an insoluble salt is formed. |
|
|
Define precipitate. |
An insoluble solid formed by a chemical reaction. A reaction between 2 soluble salts. |
|
|
What reactants are needed to make lead iodide? |
Sodium iodide and lead nitrate |
|
|
How do you seperate insoluble salt from solution? |
Filterating |
|
|
How do you remove impurities from the salt? |
Heat with bunsen. |
|
|
a diagram to make insoluble salts: |
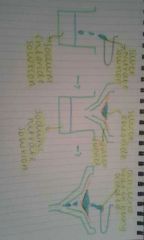
|
|
|
Name the reactants to making copper sulphate salt. |
Copper oxide and sulphuric acid |
|
|
Reaction of Copper sulphate salt - word equation |
Copper oxide + sulphuric acid ➡copper sulphate + water |
|
|
Why do you warm sulphuric acid? |
So it can disappear |
|
|
Makig insoluble salts Why do you add excess copper oxide? |
So it can have a full reaction |
|
|
Making insoluble salt what colour will the mixture be after adding copper oxide? |
Blue |
|
|
Making insoluble salts How do you know the solution is saturated? |
Until it cannot dissolve anymore |
|
|
Making insoluble salts How do you get rid of excess copper oxide? |
Filter the liquid |

