![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
17 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Boiling point |
At the boiling point the particles have enough kinetic energy to overcome the forces holding them together. |
Bonds forces |
|
|
Melting point |
At the melting point particles have enough kinetic energy to overcome the forces holding them together. |
Bonds forces |
|
|
Solute |
A substance that dissolves is called a solute |
Dissolves |
|
|
Solvent |
A substance that dissolves a solute is called a solvent. |
Dissolver |
|
|
Solution |
When a solute dissolves in a solvent it forms a solution |
2 things together |
|
|
Sublimation |
When a substance changes directly from a solid to a gas without going through the liquid stage. |
Misses out the middle |
|
|
Evaporation |
The change of state from a liquid to a gas at a temperature below its boiling point |
In between 2 and 3 |
|
|
Solidifying |
The change of state between a liquid to a solid on cooling |
2 to 1 |
|
|
Solid |
Particles are close Regular arrangement Tiny vibrations Strong bonds
|
Solid |
|
|
Endothermic |
Heat is absorbed in and used to rebuild bonds |
On cooling |
|
|
Exothermic |
Heat is given out and bonds are broken |
On heating. |
|
|
Liquid |
Particles are close Irregular shape - fills container Flowing movement Medium bonds |
Liquid |
|
|
Impurities |
Impurities raise the boiling point of a liquid and lower the melting point. |
Impurities |
|
|
Gas |
Particles are far apart Irregular arrangement Move very quickly, bouncing off each other Very weak (negligible) bonds |
Gas |
|
|
Heating curve |
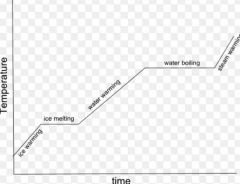
|
Heating curve. |
|
|
Expansion |
When particles gain more energy they move further apart. |
((●))((●)) ((●))((●)) |
|
|
Cooling curve |
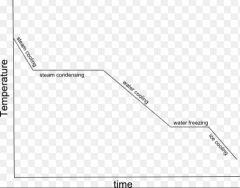
|
Cooling curve |

