![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
73 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Phase changes |

H+
|
|
|
|
Lithium |
LI+ |
|
|
|
Sodium |
Na+ |
|
|
|
Potassium |
K+ |
|
|
|
Rubidium |
Rb+ |
|
|
|
Cesium |
Cs+ |
|
|
|
Silver |
Ag+ |
|
|
|
Magnesium |
Mg2+ |
|
|
|
Calcium |
Ca2+ |
|
|
|
Strontium |
Sr2+ |
|
|
|
Barium |
Ba2+ |
|
|
|
Zinc |
Zn2+ |
|
|
|
Iron (II) |
Fe2+ |
|
|
|
Iron (!!!) |
Fe3+ |
|
|
|
Aluminum |
Al3+ |
|
|
|
Ammonium |
NH4+ |
|
|
|
Fluoride |
F- |
|
|
|
Chloride |
Cl- |
|
|
|
Bromide |
Br- |
|
|
|
Iodide |
I- |
|
|
|
Oxide |
O2- |
|
|
|
Sulfide |
S2- |
|
|
|
Nitride |
N3- |
|
|
|
Phosphide |
P3- |
|
|
|
Hydroxide |
OH- |
|
|
|
Cyanide |
CN- |
|
|
|
Nitrate |
NO3- |
|
|
|
Acetate |
C2H3O2- |
|
|
|
Sulfate |
SO4 2- |
|
|
|
Hydrogen sulfate (bisulfate) |
HSO4- |
|
|
|
Carbonate |
CO3 2- |
|
|
|
Hydrogen Carbonate |
HCO3- |
|
|
|
Phosphate |
PO4 3- |
|
|
|
Chlorate |
ClO3- |
|
|
|
Perchlorate |
ClO4- |
|
|
|
1 atm |
=760 mmHg =760 torr |
|
|
|
Boyle's Law |
Volume, of fixed quantity of gas at constant Temp, is inverse proportional to Pressure. (CONSTANT n + T) V=K*1/P PV=K P1V1=P2V2 |
|
|
|
Charles Law |
Volume, of fixed amount of gas at constant pressure, directly proportional to absolute Temp. (CONSTANT n + P) V=K*T V/T=K V1/T1=V2/T2 |
|
|
|
Avogardro's Law |
Volume, of gas at constant T and constant P is direct proportional to # moles of gas (CONSTANT T + P) V=K*n V/n=K V1/n1=V2/n2 |
|
|
|
Ideal Gas Law |
PV=nRT |
|
|
|
Ideal Gas defined |
hypothetical gas that obeys Kinetic Molecular Theory and Ideal Gas Law |
|
|
|
STP |
I atm 0*C |
|
|
|
Combined Gas Law |
# moles constant. Doesnt apply with chemical reactions P1V1/T1 = P2V2/T2 |
|
|
|
Combined Gas Law #n & V constant |
P1/T1=P2/T2 |
|
|
|
Combined Gas Law Constant T |
P1V1=P2V2 |
|
|
|
Combined Gas Law Constant Pressure |
V1/T1=V2/T2 |
|
|
|
Ideal Gas determine Density / Molar Mass of Gas |
D = P * molar / mass ---------------------- R * Temp |
|
|
|
If molar mass and Pressure is constant |
T increase D decrease |
|
|
|
Daltons Law of Partial Pressure |
total pressure of mix of gases equal sum of pressure that each exert alone Ptotal = P1 + P2 + P3 + ..... |
|
|
|
Gramham's Law of Effusion |
rate of effusion of gas inverse to square root of its molar mass r1 = 1 / square root (fancy M) |
|
|
|
lower molar mass |
balloons go down |
|
|
|
higher molar mass |
balloon go up |
|
|
|
Diffusion |
spread 1 substance through out another |
|
|
|
mole fraction (x) |
ration of number of moles of one component to total number moles in mix Xa = mol a / total mol |
|
|
|
Partial Pressure |
Pa = Xa Ptotal |
|
|
|
DeltaH |
Q phase change = DeltaH phase change * # moles |
|
|
|
Heat of fusion |
Change required to change sold at its melting point to a liquid at the same temp |
|
|
|
Heat of fusion |
Change required to change sold at its melting point to a liquid at the same temp |
|
|
|
Heat of vaporization |
Change required to change a liquid at its boiling point to a gas at the same temp |
|
|
|
Heat of sublimation |
Change required to transform a solid directly into the gas phase |
|
|
|
Phase change |
Converting from one physical state to another requires the molecules to gain enough kinetic energy to overcome the intermolecular forces |
|
|
|
Calculate amount of heat needed when the temperature changes |
Q= C x mass c DeltaTemp |
|
|
|
Calc amount of heat needed during phase change |
Q = DeltaH x moles |
|
|
|
Phase change chart |
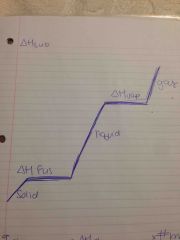
Back (Definition) |
|
|
|
Phase diagram |
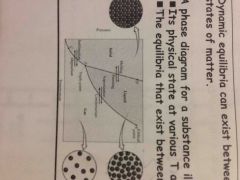
Back (Definition) |
|
|
|
Ideal gas equation |

|
|
|
|
Combined gas law |

|
|
|
|
Kinetic molecular theory |

|
|
|
|
Real gases |

|
|
|
|
Exotermic reaction |
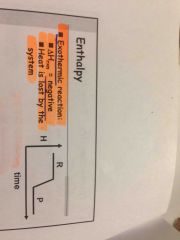
reaction |
|
|
|
Endothermic reaction |

|
|
|
|
Phase changes |

Back (Definition) |
|
|
|
J |
J |
H |

