![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
33 Cards in this Set
- Front
- Back
|
Democritus |
Suggested that everything was composed of minute, indivisible, indestructible particles of pure matter 450 BCE |
|
|
Mendeleev |
Period law |
|
|
Alkanes |
Hydrocarbons with only single bonds between atoms Examples: Methane, Ethane and Propane Methane is the simplest Hydrocarbon/alkane |
|
|
Alkenes |
Hydrocarbons with one double bond between two carbon atoms Examples: Ethene, Propene and Butene Classified as unsaturated (carbon-based compounds containing double or triple bonds between carbon atoms) hydrocarbons Double bond between two of the carbon atoms means that alkenes contain less hydrogen than the maximum amount possible |
|
|
Alkynes |
Each compound in the alkyne series has one triple bond between carbon atoms Ethyne is the smallest alkyne |
|
|
Rules for Naming Carbon Compounds |
• The longest unbranched chain containing the functional group is the parentmolecule, or simply the longest unbranched chain for alkanes. Remember that, thelongest chain can go round a bend. • Indicate the position of the functional group with a number, numbering from the endnearest the functional group. • Name the branches, and indicate the number of branches. • The prefix 'di' indicates there are 2 branches. • The prefix 'tri' indicates there are 3 branches. • Ethyl indicates there are 2 carbon atoms in the branch. • Methyl indicates there is 1 carbon atom in the branch |
|
|
Avagadro's Constant |
NA = 6.02 x 10^23 mol-1 |
|
|
Relative Atomic Mass Formula |
Ar = (relative isotopic mass1 x % abundance) + (relative isotopic mass2 x % abundance) + ... ______________________________________________________ 100 |
|
|
Finding the number of particles: |
N = n x NA |
|
|
Amount of substance (in mol) in a given substance |
n = N ÷ NA |
|
|
Finding the mols/mass/molar mass |
n = m ÷ M |
|
|
Mole in a solution |
n = c x v |
|
|
To find the mass |
m = n x M |
|
|
Ionic compounds |
Ioniccompounds are made up of ions! This is bonding between Metal Cations andNon-Metal Anions |
|
|
Ionic properties |
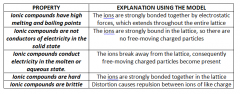
|
|
|
Ionic bonding model |
- Positive ions and negative ions are strongly bonded ina regular lattice called an ionicnetwork lattice - The strong bonding results from the electrostatic attraction between oppositelycharged ions - The ions are arranged so that positively charged ions are surrounded by negatively charged ionsand vice versa. - Therelative number of cations and anions is fixed by the requirement that thesolid has a neutral charge |
|
|
Metals always |
form positive ions |
|
|
Non-metals always |
from negative ions |
|
|
Metal atoms release electrons |
that are taken up by non-metalatomsThe positive metal ions are attracted to the negativenon-metal ions producing crystals. This structure is called an ionic network lattice. |
|
|
Ionic substances in aqueous solutions |
When anionic substance is in an aqueous solution, or in the molten state; the positiveand negative ions DISSOCIATE and are left to move freely through the solution.Because there are now free-moving charged particles, the ions are able to movearound in order to allow a charge to pass through. |
|
|
Metallic Bonding |

|
|
|
Metallic Bonding Model |
Metal atoms let looseouter shell electrons and form positiveions The cations arearranged in a regular, three-dimensional lattice The outer shellelectrons are no longer tied up to any atom in particular atom. They are calleddelocalised electronsand are free to move throughout the lattice. The metalliccations are held in the lattice by the electrostaticattraction to the delocalised electrons |
|
|
Chemical Reactivity of metals |
Metals tendto react by losing electrons. The chemical reactivity of a metal depends on howeasily electrons can be removed from its atoms. The lower the amount of energyrequired to remove electrons from the atom, the more reactive the atom is. |
|
|
Limitations of the metallic bonding model? |
The model cannot explain why some metals are magneticand some are not Why densities and melting temperatures of metals varyso much |
|
|
Modifying Metals |
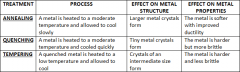
|
|
|
Nobel Gases |
Most unreactive non-metals All except helium, have 8 valence electrons – they possess a stable octet structure. At room temperature, all the noble gases exist as colourless monoatomic gases (insoluble in water) (e.g. argonconsists of free atoms, Ar and not Ar2.) Helium used in weather balloons and airships Neon used in advertising lights Argon commonly used to fill light (filament) bulbs |
|
|
Periodic Table Trends |
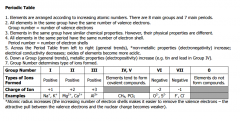
|
|
|
Dalton's atomic theory |
1. All matter is composed of atoms, held togetherby forces of attraction. 2. Atoms are indivisible and cannot be created nordestroyed. 3. All atoms of the same element are identical. 4. All atoms of different elements are totallydifferent. 5. Atoms of different elements can combined to formsimple whole number compounds. |
|
|
Thomson Atomic theory |
Plum Pudding Model Protons and Electrons randomly scattered throughoutthe atom. Cathode rays. Cathode Rays: Alight is produced when a current is passed through a tube, streaming from thecathode to the anode. These cathode rays are made of negatively chargedparticles moving in straight lines, unless subject to magnetic or electricalforce. |
|
|
Rutherford Atomic theory |
Introduction of the nucleus, a concentrated region of positive charge. Electrons orbit around the nucleus in random orbits. α-particle scattering:α-particles are made of positively charged helium ions. These particleswould scatter and even rebound backwards if shot at a gold sheet. Only a highlyconcentrated area of positive charge could do this, hence, a nucleus. Nuclear Model |
|
|
Bohr Atomic Theory |
Electron Shell Electron shells. Each holds 2n2 electronswhere n is the shell number. Emission Spectra: Whenphotons are emitted, they can be divided into their individual wavelengths witha spectroscope. This produces an emission spectrum. Each element has a uniqueemission spectrum. |
|
|
Schrodiner Atomic Theory |
Mechanical Model Subshells, three-dimensional orbits. Clusters of lines in emission spectra |
|
|
Isotope |
atoms of the same element that have different atomic weights due to the differing amounts of neutrons |

