![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
27 Cards in this Set
- Front
- Back
|
If the formula for sulfuric acid is h2so4, what would be the expected formula for the compound between hydrogen and tellurium, Te? |
H2TeO4 |
|
|
Which of the following subshells has the higher energy |
4f |
|
|
Suppose an electron moves from the second shell to the third shell |
The move required an input of energy |
|
|
The total number of f orbital in an F subshell is |
7 |
|
|
What is the maximum number of electrons that can occupy the third shell |
18 |
|
|
How many electrons are in the outer shell of element 15 |
5 |
|
|
What is the shell number for the outer shell electrons in bromine, br? |
4 |
|
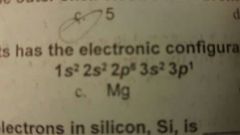
Which of the following about has an electronic configuration shown here? |
Al |
|
|
The total number of unpaired electrons in Silicon,si, is |
2 |
|
|
Which of the following will not have electrons in the third orbital? |
Na+ |
|
|
What type of electron is the distinguishing electron in s? |
P |
|
|
Which of the following distinguishing electrons represents an element with the properties similar to an element with a 3p3 distinguishing electron? |
4p3 |
|
|
Metalloids can express the characteristics of both metals and nonmetals. Which of the elements below is more likely to have the characteristics of a metal than a non-metal? |
Sb |
|
|
Which of the following elements is classified as a representative metal? |
Element 38 |
|
|
Which of the following elements is a non-metal? |
I |
|
|
Which of these elements is a gas at room temperature |
Nitrogen |
|
|
Which element has the distinguishing electron, 5p4 |
Te |
|
|
The radius of a k atom is____ a Ca atom |
Larger than |
|
|
The highest energy shell for an element that contains electrons is known as the |
Valence shell |
|
|
_____ states that, electrons will not join other electrons in an orbital if an empty orbital of the same energy is available |
Hunds rule |
|
|
_____ explains why orbitals can contain a maximum of two electrons |
Pauli Exclusion Principle |
|
|
Choose the correct electron figuration for the element As |

|
|
|
Which of the following is a reasonable explanation as to why atomic radii become smaller as one moves to the right for the representation elements in a period? |
The charge of the nucleus increases, pulling the shells closer |
|
|
Which of the following violates the Pauli Exclusion Principle |

|
|
|
Which of the following is the correct valence electron configuration of phosphorus? |

|
|
|
Which pair of the following represents the same electron configuration |

|
|
|
What is the basis for placing groups of elements into a family on the periodic table? |
Similar valence electron configurations |

