![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
26 Cards in this Set
- Front
- Back
|
When do zinc/copper carbonate decompose?
|
When heated
|
|
|
How do you know when zinc/copper carbonate are decomposing? Give an example.
|
Distinctive colour changes
e.g. ZnCO₃ (s) → ZnO(s) + CuCO₃(s) → CuO(s) + CO₂(g) White Yellow Solid (hot) Green Black Solid White solid (cold) Solid Solid |
|
|
When does Carbon dioxide dissolve well in water?
|
When it is under pressure.
|
|
|
Why can CO₂ be collected by downward delivery?
|
It is more dense than air
|
|
|
What is downward delivery?
|
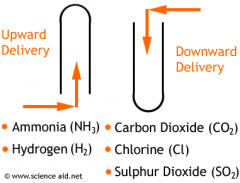
|
|
|
When does Carbon Dioxide become less soluble?
|
At low pressure or high temperature
|
|
|
Why is carbon dioxide used in fire extinguishers?
|
It displaces oxygen because it is more dense than air, starving the flames
|
|
|
What does Acid + metal create?
|
Acid + Metal → Salt + Hydrogen
|
|
|
How do you prepare hydrogen?
|

|
|
|
Why doesn't Aluminium readily react with dilute acids?
|
It has a protective oxide layer
|
|
|
What happens if Aluminium is reacted with dilute acid? Give 2 symbol equations
|
2Al(s) + 6HCl(aq) → 2AlCl₃(aq) + 3H₂(g)
2Al(s) + 3H₂SO₄(aq) → Al₂(SO₄)₃(aq) + 3H₂(g) |
|
|
What happens when a metal corrodes? Give an example.
|
Metal reacts with oxygen
e.g. Magnesium + Oxygen → Magnesium Oxide |
|
|
What is given off when a metal reacts with water?
|
Hydrogen
|
|
|
What do less reactive metals react with steam to form?
|
metal oxides
|
|
|
What do more reactive metals react with steam to form?
|
metal hydroxides
|
|
|
What is given off when a metal reacts with acid? Give an example.
|
Hydrogen and a salt
Magnesium + Hydrochloric→ Magnesium + Hydrogen acid chloride |
|
|
What is the test for hydrogen?
|
A lit splint will pop in its presence
|
|
|
What is a displacement reaction?
|
A more reactive metal displaces a less reactive metal from its compound
|
|
|
When will a reactive metal displace a less reactive one from a metal oxide? Give an example.
|
When it is heated.
e.g. Zinc + Iron Oxide → Iron + Zinc Oxide |
|
|
What is a Thermit reaction?
|
A very exothermic reaction
|
|
|
What is an exothermic reaction?
|
A reaction with the evolution of heat
|
|
|
What is a reducing agent?
|
The element that takes oxygen from a compound
|
|
|
What is an oxidising agent?
|
The element that gives oxygen to a reducing agent
|
|
|
When is rust formed?
|
When iron reacts with water and oxygen
|
|
|
Name 4 ways of preventing rust.
|
Regular painting or oiling
Galvanising (coat it with zinc) Make object out of non rusting object Attach zinc bars to ships |
|
|
What is zinc used as in method 2 and 4 of Q25?
|
A sacrificial metal
|

