![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
7 Cards in this Set
- Front
- Back
|
Neutron |
|
|
|
Isotopes |
|
|
|
Atomic Theory |
i. atom is the smallest unit that can participate in a chemical change 2. Elements are composed of atoms i. all atoms of the same element are alike ii. all atoms of other elements are different 3. Compound is composed of 2+ elements combined in small whole # ratio's Atoms can not be created or destroyed in a chemical change-- the atoms are just rearranged |
|
|
Law of Multiple Proportions |
When 2+ elements react to form more than 1 compound- a fixed mass of one element will react with masses of the other element in small whole # ratio's |
|
|
Cathode Ray Tube |
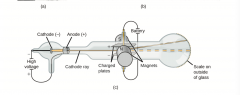
|
|
|
Oil Drop Experiment |
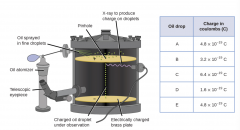
|
|
|
Gold Foil Experiment |
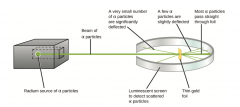
|

