![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
160 Cards in this Set
- Front
- Back
|
If something is sour, it is probably a what? The more _________ the thing, the more sour the taste. |
An acid The more acidic the thing, the more sour the taste. |
|
|
T/F Acids do not dissolve metals. |
False, acids dissolve many metals. |
|
|
The sour taste of _________, _________, _______________ and _____________ is due to their acid content. |
The sour taste of lemons, vinegar, plain yogurt and vitamin C is due to their acid content. |
|
|
Acids react with ________ to form _______ and a _______ through __________________ reactions. |
Acids react with bases to form water and a salt through neutralization reactions. |
|
|
*A neutralization reaction between HCl and NaOH forms what? HCl + NaOH --> ______ + _______ Which of the reactants is the acid and which is the base? |
HCl + NaOH --> H2O + NaCl Acid = HCl Base = NaOH |
|
|
Litmus paper turns what color in acids? Litmus paper turns what color in bases? |
Acid = Red Base = Blue |
|
|
*What are the four main properties of acids? |
Acids dissolve many metals. Acids have a sour taste. Acids react with bases to form water and a salt. Acids turn litmus paper red. |
|
|
In concentrated form, many acids are ___________. If spilled on clothing, they _________ the clothing material. If they contact the skin, they produce severe ________. If ingested, concentrated acids will __________ the mouth, throat, stomach, and gastrointestinal tract. In larger amounts, they can _______. |
In concentrated form, many acids are dangerous. If spilled on clothing, they dissolve the clothing material. If they contact the skin, they produce severe burns. If ingested, concentrated acids will damage the mouth, throat, stomach, and gastrointestinal tract. In larger amounts, they can kill. |
|
|
Limes and lemons are high in what content? |
Acid content. |
|
|
*What are the six strong bases? |
Lithium hydroxide Sodium hydroxide Potassium hydroxide Calcium hydroxide Strontium hydroxide Barium hydroxide |
|
|
*What is Lithium hydroxide's chemical formula? |
LiOH |
|
|
*What is Sodium hydroxide's chemical formula? |
NaOH |
|
|
*What is Potassium hydroxide's chemical formula? |
KOH |
|
|
*What is Calcium hydroxide's chemical formula? |
Ca(OH)2 |
|
|
*What is Strontium hydroxide's chemical formula? |
Sr(OH)2 |
|
|
*What is Barium hydroxide's chemical formula? |
Ba(OH)2 |
|
|
*What are the six strong acids? |
Hydrochloric Acid Hydrobromic Acid Hydroiodic Acid Nitric Acid Perchloric Acid Sulfuric Acid |
|
|
*What is Hydrochloric Acid's chemical formula? |
HCl |
|
|
*What is Hydrobromic Acid's chemical formula? |
HBr |
|
|
*What is Hydroiodic Acid's chemical formula? |
HI |
|
|
*What is Nitric Acid's chemical formula? |
HNO3 |
|
|
*What is Perchloric Acid's chemical formula? |
HClO4 |
|
|
*What is Sulfuric Acid's chemical formula? |
H2SO4 |
|
|
Which property is not generally associated with acids? a. Sour Taste b. Volatility c. Ability to neutralize bases d. Ability to dissolve metals |
b. Volatility |
|
|
*What are the four main properties of bases? |
Bases feel slippery. Bases taste bitter. Bases react with acids to form water and a salt. Bases turn litmus paper blue. |
|
|
*A neutralization reaction between KOH and H2SO4 forms what? 2KOH + H2SO4 --> ______ + _______ Which of the reactants is the acid and which is the base? |
2KOH + H2SO4 --> 2H2O + K2SO4 Base = KOH Acid = H2SO4 |
|
|
The bitter taste of ________, _________________, and some ___________ is due to their base content. |
The bitter taste of coffee, milk of magnesia, and some medicines is due to their base content. |
|
|
*Bases react with ________ to form _______ and a _______ through __________________ reactions. |
Bases react with acids to form water and a salt through neutralization reactions. |
|
|
Sodium bicarbonate, also known as _______________, is a common _________ and is found in many __________ products. |
Sodium bicarbonate, also known as baking soda, is a common antacid and is found in many cleaning products. |
|
|
In concentrated forms, many bases are ______________ and will ______ the skin on contact. If ingested, concentrated bases _________ the mouth, throat, and gastrointestinal tract. |
In concentrated forms, many bases are dangerous and will burn the skin on contact. If ingested, concentrated bases damage the mouth, throat, and gastrointestinal tract. |
|
|
*Write a chemical equation to show the neutralization of nitric acid (HNO3) by potassium hydroxide (KOH). |
HNO3 + KOH --> H2O + KNO3 acid base water salt |
|
|
*All neutralization equations have what basic form? |
Acid + Base --> Water + Salt |
|
|
*Write a chemical equation to show the neutralization of sulfuric acid (H2SO4) by sodium hydroxide (NaOH). |
H2SO4 + 2NaOH --> 2H2O + Na2SO4 acid base water salt |
|
|
*What is an Arrhenius Acid? |
A substance that produces hydrogen ions (H+) in solution. |
|
|
*What is an Arrhenius Base? |
A substance that produces hydroxide ions (OH-) in solution. |
|
|
*T/F The Arrhenius definition of acids and bases apply in all cases. |
False, Although the Arrhenius definition of acids and bases describes a great deal of acid-base chemistry, it does not apply in all cases. |
|
|
*Ammonia (NH3) does not contain OH-. Is it a base? |
Yes |
|
|
*NH3 + H2O --> _______ + _______ |
NH3 + H2O --> NH4+ + OH- |
|
|
T/F In the Arrhenius definition, ammonia produces OH- when it is combined with water. |
True |
|
|
*There are two definitions of acids and bases. What are they? Which one is a broader definition? |
The Arrhenius definition of acids and bases. The Bronsted-Lowry definition of acids and bases. The Bronsted-Lowry definition is broader. |
|
|
T/F The Bronsted-Lowry definition applies more naturally to NH3 and works in solutions that do not contain water. |
True |
|
|
*What is a Bronsted-Lowry acid? |
A proton donor |
|
|
*What is a Broasted-Lowry base? |
A proton acceptor |
|
|
*The Bronsted-Lowry definition of acids and bases focuses on what? |
The transfer of H+ ions (protons). |
|
|
*Draw the equation for the dissociation of HCl in water. In this equation, what acts as the acid and as the base? |
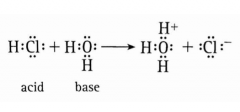
Acid = HCl Base = Water |
|
|
*Draw the equation for the dissociation of NH3 in water. In this equation, what acts as the acid and as the base? |
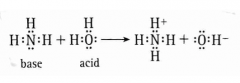
Acid = Water Base = NH3 |
|
|
T/F The Bronsted-Lowry definition is broader and allows more substances to be considered acids and bases. |
True |
|
|
*Identify the Bronsted-Lowry acid and base in the following reaction: CH3COOH + H2O --> CH3COO- + H3O+ |
Acid = CH3COOH Base = Water |
|
|
*Identify the Bronsted-Lowry acid and base in the following reaction: C5H5N + H2O --> C5H5NH+ + OH- |
Acid = Water Base = C5H5N |
|
|
*Write the chemical formula for the dissociation of HCl in water. |
HCl + H2O --> H3O+ + Cl- |
|
|
*Donating a proton generally means what? |
Giving away a Hydrogen. |
|
|
*What are strong acids? What is an example of a strong acid? |
Acids that completely dissociate in solutions. HCl |
|
|
*What are weak acids?
What is an example of a weak acid? |
Acids that do not completely dissociate in solutions.
CH3COOH |
|
|
*What does a double arrow mean in a chemical reaction formula? |
Incomplete dissociation (a weak acid) |
|
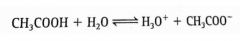
*What does this equation mean? |
The incomplete dissociation of CH3COOH in a solution. CH3COOH is a weak acid. The solution contains significant amounts of CH3COOH, H3O+, and CH3COO-. |
|
|
T/F Because HCl is a strong acid, it completely dissociates in solution. This is shown with a single arrow in the chemical reaction formula. |
True |
|
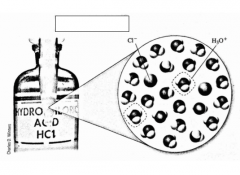
Complete the example. |
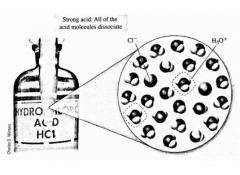
|
|
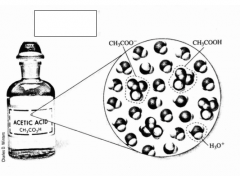
Complete the example. |

|
|
|
*What are strong bases? What is an example of a strong base? |
Bases that completely dissociate in solution. NaOH |
|
|
*What are weak bases?
What is an example of a weak base? |
Bases that do not completely dissociate in solution, or bases that work by ionizing water.
NH3 |
|
|
What is NH3? |
Ammonia |
|
|
Show the dissociation of NaOH in solution. |
NaOH --> Na+ + OH- |
|
|
*Show the incomplete dissociation of NH3 in water. |
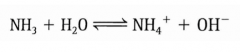
|
|
|
*A solution of NaOH contains what and what but not what? |
A solution of NaOH contains Na+ and OH- but no NaOH? |
|
|
The double arrow in the Ammonia reaction means what? |
The reaction does not go to completion. |
|
|
HF is a weak acid. Which statement is true for an HF solution? a. All of the HF molecules dissociate in solution. b. Some of the HF molecules dissociate in solution. c. None of the HF molecules dissociate in solution. |
b. Some of the HF molecules dissociate in solution. |
|
|
*The acidity of a solution is normally specified by the concentration of what? |
Of H3O+ (concentration of hydronium) in moles per liter (M). |
|
|
*T/F The concentration of H3O+ in an acid solution will not necessarily be equal to the concentration of the acid itself; it depends on whether the acid is strong or weak. |
True |
|
|
A 1 M HCl solution (strong acid) will have an H3O+ concentration of ___ M, but a 1 M CH3COOH solution (weak acid) will have an H3O+ concentration significantly ______ ___ M. |
A 1 M HCl solution (strong acid) will have an H3O+ concentration of 1 M, but a 1 M CH3COOH solution (weak acid) will have an H3O+ concentration significantly below 1 M. |
|
|
T/F We usually specify the acidity of a solution by referring to the H3O+ concentration directly rather than the concentration of the acid that formed it. |
True |
|
|
*[H3O+] = |
= concentration of H3O+ in M. |
|
|
*Pure water at 25º C naturally contains [H3O+] = |
= 1 X 10^-7 M (0.0000001 M) |
|
|
Water shows slight acid-base behavior. Draw that equation. |
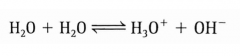
|
|
|
*What is the name for H3O+? |
Hydronium |
|
|
*A solution is considered acidic if it has what? A solution is considered basic if it has what? |
[H3O+] > 1 X 10^-7 M [H3O+] < 1 X 10^-7 M |
|
|
What is the pH scale? What is neutral on the pH scale? What is acidic on the pH scale? What is basic on the pH scale? |
A scale that expresses acidity or basicity in a compact way. Neutral = pH of 7 Acidic = pH less than 7 Basic = pH greater than 7 |
|
|
*For every change of one unit on the pH scale, the [H3O+] concentration changes by a factor of what? |
By a factor of 10. |
|

Complete the tables. |
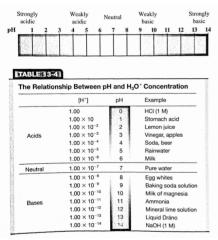
|
|
|
The ideal pH of a swimming pool is 7.2. You measure the pH of your pool to be 6.5. What should you add, acid or base, to restore your pool to the ideal pH? |
You should add a base to neutralize some of the excess acid. |
|
|
The acid responsible for the sourness of lemons, limes, oranges, and grapefruit is called what? |
Citric Acid |
|
|
T/F Citrus fruits, like many acidic foods, are resistant to spoilage because many microorganisms cannot survive the low-pH environment. |
True |
|
|
Many low-acid foods can be made spoilage resistant by storing them in ______ or by _______________ with _______-______-forming bacteria. This technique is called __________ and is often used as a way to preserve cucumbers (pickles) and cabbage (sauerkraut). |
Many low-acid foods can be made spoilage resistant by storing them in acid or by fermentation with lactic-acid-forming bacteria. This technique is called pickling and is often used as a way to preserve cucumbers (pickles) and cabbage (sauerkraut). |
|
|
_______ acid is formed in wine when the wine is exposed to _________ for an extended period. The presence of _______ acid in a newly opened wine bottle indicates _________ storage. |
Acetic acid is formed in wine when the wine is exposed to oxygen for an extended period. The presence of acetic acid in a newly opened wine bottle indicates improper storage. |
|
|
_______ acid is also produced by certain _______ that metabolize _______ and produce _______ acid as a product. |
Acetic acid is also produced by certain yeasts that metabolize sugars and produce acetic acid as a product. |
|
|
__________ acid, originally obtained from the bark of the willow tree, is the parent molecule for _________________ acid, or __________, the most widely used of all drugs. |
Salicylic acid, originally obtained from the bark of the willow tree, is the parent molecule for acetylsalicylic acid, or aspirin, the most widely used of all drugs. |
|
|
Hydrochloric acid is found in high concentrations where? |
In the stomach. |
|
|
Phosphoric acid (H3PO4) is often added to what to impart what? |
Softdrinks and beer Tartness |
|
|
Carbonic acid is produced by what? and is present in what? |
The reaction between carbon dioxide and water. All carbonated beverages. |
|
|
All _____ contain _____, with concentrations ranging between 0.60 and 0.80% of the total _____ volume. |
All wines contain acid, with concentrations ranging between 0.60 and 0.80% of the total wine volume. |
|
|
The acids in wine come from two different sources. What are they? |
The grapes themselves The fermentation process, in which bacteria convert sugars into ethyl alcohol and carbon dioxide, producing or modifying acids in the process. |
|
|
The citrus flavor in wine is due to what? |
Citric Acid |
|
|
The apple flavor in wine is due to what? |
Malic Acid |
|
|
The buttery flavor in wine is due to what? |
Lactic Acid |
|
|
Bases constitute the active ingredients in what? |
Antacids |
|
|
The often disagreeably _______ taste associated with _______ may be an evolutionary adaption that warns against __________, a family of organic, nitrogen-containing ______ that are often poisonous. A few alkaloids are ___________, ___________, __________, and _________. |
The often disagreeably bitter taste associated with bases may be an evolutionary adaption that warns against alkaloids, a family of organic, nitrogen-containing bases that are often poisonous. A few alkaloids are caffeine, morphine, nicotine, and cocaine. |
|
|
*Most antacids contain one or more of the following bases: |
Sodium Bicarbonate (NaHCO3) Calcium Carbonate (CaCO3) Magnesium Carbonate (MgCO3) Magnesium Hydroxide (Mg(OH)2) Aluminum Hydroxide (Al(OH)3) |
|
|
*The antacids mentioned dissociate in water to produce what? |
A metal ion and a base. |
|
|
What is the active ingredient in Alka-Seltzer?
What is it also sold as?
People with what should avoid this. |
Sodium bicarbonate
Baking soda
Hypertension, because of the sodium content. |
|
|
What is the active ingredient in Tums? |
Calcium Carbonate |
|
|
What is the active ingredient in milk of magnesia? |
Magnesium Hydroxide |
|
|
What are the active ingredients in Mylanta? What do they tend to do to each other? |
Magnesium Hydroxide and Aluminum Hydroxide. Have a canceling effect. Mg(OH)2 = Laxative Al(OH)2 = Constipating |
|
|
T/F Bases are found in several household cleaning products. |
True |
|
|
What is lye another name for? |
Sodium hydroxide |
|
|
What is the active ingredient in drano? And what does it do? |
Sodium Hydroxide It dissolves hair and grease. |
|
|
T/F Many baked goods are made with leavening agents that produce pockets of carbon dioxide gas in the dough. What is the ingredient used to produce the carbon dioxide? |
True Baking powder |
|
|
*Sulfur and nitrogen impurities present in fossil fuels - especially coal - form gaseous _____ and _____ during combustion. These gases combine with atmospheric water and oxygen to form _________ acid and _______ acid, which then fall as acid rain. |
Sulfur and nitrogen impurities present in fossil fuels - especially coal - form gaseous SO2 and NO2 during combustion. These gases combine with atmospheric water and oxygen to form sulfuric acid and nitric acid, which then fall as acid rain. |
|
|
*Draw the equation for SO2 produced acid rain. Draw the equation for NO2 produced acid rain. |
2SO2 + O2 + 2H2O --> 2H2SO4 4NO2 + O2 + 2H2O --> 4HNO3 |
|
|
We might expect pure, unpolluted rain to have a pH of 7.0, but it does not. Rain is naturally slightly acidic because of the presence of carbon dioxide in the atmosphere. Carbon dioxide combines with water to form carbonic acid, a weak acid. Draw the reaction. |
CO2 + H2O --> H2CO3 |
|
|
*The pH of water saturated with CO2 is about ____. The pH of rain in the United States varies from ____ to ____, more acidic than would be expected from CO2 alone. Where does most acidic rain occur in the United States? |
The pH of water saturated with CO2 is about 5.6. The pH of rain in the United States varies from 4.6 to 6.1, more acidic than would be expected from CO2 alone. The northeastern United States. |
|
|
*Most of the acidity beyond what is expected from CO2 is attributed to what? |
Fossil-fuel pollutants |
|
|
*What are the four main negative effects of acid rain? |
Damage to lakes and streams Damage to building Materials Damage to Forests Reduced Visibility |
|
|
Congress targeted acid rain by doing what, in what year? What was the goal of this? |
Amending the Clean Air Act in 1990. To cut electric utilities sulfur oxide emissions in half. |
|
|
In 2005, the EPA introduced this rule which requires 28 states in the eastern United States to further reduce their SO2 and NO2 emissions. |
The Clean Air Interstate Rule |
|
|
*What are four ways Utilities can cut emissions? |
-Use low-sulfur coal -Remove sulfur from coal -Use Flue gas scrubbers to remove the sulfur dioxide from the exhaust -Encourage conservation and efficiency programs for their customers. |
|
|
T/F Acids always have Hydrogen in front. T/F Bases always have OH at the end. |
True (in most cases) True (in most cases) |
|
|
When talking about strong acids and strong bases, the word strong relates to what? |
Dissociation |
|
|
*[H3O+] + [OH-] is always equal to what? |
[H3O+] + [OH-] = 1 X 10^-14 |
|
|
*pH + pOH = |
= 14 |
|
|
*[H+] = |
= (1 X 10^-14) / [OH-] |
|
|
*[OH-] = |
= (1 X 10^-14) / [H+] |
|
|
*pOH = |
= -log[OH-] |
|
|
*pH = |
= -log[H3O+] |
|
|
The Ion product of water Kw is two things. What are they? |
Inversely proportional The product is always the same |
|
|
T/F Acids ionize in water to produce H+ions and anions. T/F Bases dissociate in water to produce OH-ions and cations. T/F Strong acids = 100% ionization |
True True True |
|
|
*What is the name of this chemical: LiOH |
Lithium hydroxide |
|
|
*Is LiOH a strong acid or a strong base? |
Strong Base |
|
|
*Is Lithium hydroxide a strong acid or a strongbase? |
Strong Base |
|
|
*What is the name of this chemical: NaOH |
Sodium hydroxide |
|
|
*Is NaOH a strong acid or a strong base? |
Strong Base |
|
|
*Is Sodium hydroxide a strong acid or a strong base? |
Strong Base |
|
|
*What is the name of this chemical: Sr(OH)2 |
Strontium hydroxide |
|
|
*Is Sr(OH)2 a strong acid or a strong base? |
strong base |
|
|
*Is Strontium hydroxide a strong acid or a strong base?
|
strong base |
|
|
*What is the name of this chemical: HBr |
Hydrobromic Acid |
|
|
*Is HBr a strong acid or a strong base? |
Strong Acid |
|
|
*Is Hydrobromic Acid a strong acid or a strong base? |
Strong Acid |
|
|
*What is the name of this chemical: H2SO4 |
Sulfuric Acid Acid |
|
|
*Is H2SO4 a strong acid or a strong base? |
Strong Acid |
|
|
*Is Sulfuric Acid Acid a strong acid or a strong base?
|
Strong Acid |
|
|
*What is the name of this chemical: KOH |
Potassium hydroxide |
|
|
*Is KOH a strong acid or a strong base? |
Strong Base |
|
|
*Is Potassium hydroxide a strong acid or a strong base? |
Strong Base |
|
|
*What is the name of this chemical: Ca(OH)2 |
Calcium hydroxide |
|
|
*Is Ca(OH)2 a strong acid or a strong base? |
Strong Base |
|
|
*Is Calcium hydroxide a strong acid or a strong base? |
Strong Base |
|
|
*What is the name of this chemical: Ba(OH)2 |
Barium hydroxide |
|
|
*Is Ba(OH)2 a strong acid or a strong base? |
Strong Base |
|
|
*Is Barium hydroxide a strong acid or a strong base? |
Strong Base |
|
|
*What is the name of this chemical: HCl |
Hydrochloric Acid |
|
|
*Is HCl a strong acid or a strong base? |
Strong Acid |
|
|
*Is Hydrochloric Acid a strong acid or a strong base? |
Strong Acid |
|
|
*What is the name of this chemical: HI |
Hydroiodic Acid |
|
|
*Is HI a strong acid or a strong base? |
Strong Acid |
|
|
*Is Hydroiodic Acid a strong acid or a strong base? |
Strong Acid |
|
|
*What is the name of this chemical: HNO3 |
Nitric Acid |
|
|
*Is HNO3 a strong acid or a strong base? |
Strong Acid |
|
|
*Is Nitric Acid a strong acid or a strong base? |
Strong Acid |
|
|
*What is the name of this chemical: HClO4 |
Perchloric Acid |
|
|
*Is HClO4 a strong acid or a strong base? |
Strong Acid |
|
|
*Is Perchloric Acid a strong acid or a strong base? |
Strong Acid |

