![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
54 Cards in this Set
- Front
- Back
|
What does the rate of reaction measure and when are they usually faster or slower? |
- the rate of reaction measures how much product is formed in a fixed period of time - reactions are usually fast at the start and then slow down as the reactants are used up |
|
|
What are the units commonly used when measuring the rates of reactions? |
- g/s (grams per second) and g/min (grams per minute) when meauring the mass of a product formed - g/s is used for faster reactions and g/min for slower reactions - cm cubed/s and cm cubed/min when meauring the volume of gas produced - cm cubed/s is used for faster reactions and cm cubed/min for slower reactions |
|
|
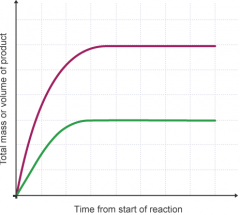
How can the rate of reaction be worked out? |
- from the gradient of a graph which can be calculated using construction lines - choose a part of the graph where it's straight and not curved - using the scales on the graph, measure the value of y and x - gradient = y/x - add units, making sure these are the same as those used on the graph |
|
|
What is the limiting reactant, and what is the relationship between the amount of product formed in a reaction and the amount of limting reactant used? |
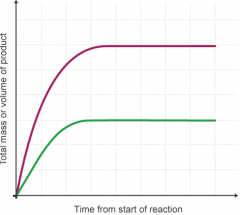
- the reactant not in excess that gets used up by the end of the reaction - the amount of product formed in a reaction is directly proportional to the amount of limiting reactant used - e.g. if the amount of limitng reactant doubles, the amount of product formed doubles |
|
|
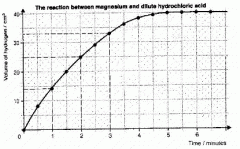
When do reactions occur and what does this mean (to do with limiting reactants), how can you estimate the mass at 15s (bottom of graph) and how can you estimate the mass at 40s? |
- when particles collide together so if the number of reacting particles of one reactant is limited, the number of collisions by particles of that reactant is limited - the mass of gas collected at 15s can be estimated by interpolation. - if the mass was not a limiting factor, the volume of gas that would be collected after 40s can be estimated by extrapolation, using the initial line |
|
|
When do chemical reactions take place and what does the rate of reaction depend on? |
- when particles collide - depends on the number of collisions between the reacting particles: the higher the number of collisions that take place, the faster the reaction |
|
|
How can the rate of reaction be increased? |
- by increasing the concentration, raising the temperature or the pressure |
|
|
How does increasing the concentration increase the rate of reaction? |
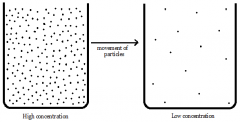
- as the concentration increases the particles become more crowded - this increases the number of collisions between reacting particles |
|
|
How does raising the temperature increase the rate of reaction? |
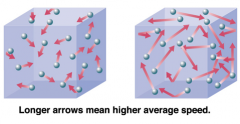
- as the temperature increases, the particles gain kinetic energy and move around more quickly, so collisions between reacting particles are more successful, and also occur in greater number |
|
|
How does increasing pressure increase the rate of reaction? |
- increases in pressure forces particles closer together, increasing the rate of reaction |
|
|
What does the rate of reaction also depend on? |
- the collision frequency: this describes the number of successful collisions between reacting particles each second |
|
|
What must happen for a successful collision to occur and describe the 3 ways in which this happens: |
- each particle must have enough energy to react - as the concentration increases, the number of collisions per second increases, and so the rate of reaction increases - as the temperature increases, the reactants have more kinetic energy, so collisions are more energetic and frequent, so more likely to be successful - increasing the pressure is gases forces particles closer together, increasing collision frequency |
|
|
How do tables and graphs show that a reaction is finished, how can they be used to compare rates of reaction and what is an essential skill? |
- when a reaction is finished on a graph the line will be horizontal and in a table the numbers will stop changing - when comparing the rates of reaction on graphs, the steeper the line, the faster the reaction and in a table, the larger the change between readings, the faster the reaction - to be able to sketch graphs to show the affect of changing temperature, concentration and pressure on: - the rate of reaction - the amount of formed in a reaction |
|
|
What do extrapolation and interpolation mean? |
- extrpolation means making an estimate beyond the range of results and to do this you should assume that the trend shown by the existing data will continue - interpolation means making an estimate between results in a range |
|
|
What can combustible powders cause, how do they react and what do factories producing them have to do? |
- explosions - owners of a factory using combustible powders such as flour, custard powder or sulfur must be very careful - they must ensure that the powders can't reach the open atmosphere and that the chances of a spark being produced near the powders is very small |
|
|
How is a surface area increased, what type of reactant has a larger surface area and what does increasing surface area do? |
- by breaking up a block into smaller pieces - a powdered reactant has a much larger surface area than the same mass of reactant in block form - as the surface area of a solid is increased so is the rate of reaction |
|
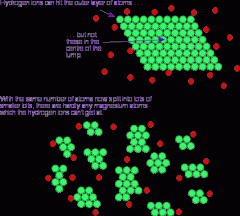
Describe the 'small surface area' diagram: |
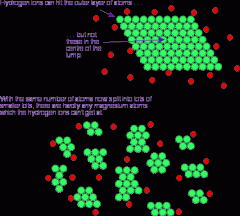
- the green particles at the top represent a solid block - the red particles represent the other reactant - most particles in the solid block can't react as collisions can only occur at the surface |
|
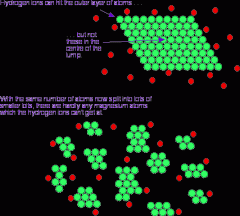
Describe the 'large surface area' diagram: |
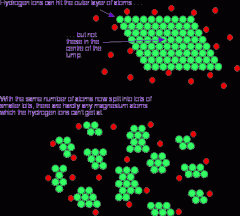
- the green particles at the bottom from the block are now spread out - more surfaces on these particles are exposed, so more collisions are possible, increasing the rate of reaction |
|
|
When do reactions occur, what can powders do, and what happens in a block of material? |
- when reactant particles collide with sufficient energy - they can spread throughout a reaction mixture, increasing the collision frequency (collisions per second) - in a block of material, collisions can only occur with the particles on the surface - most of the particles in the block are trapped on the inside, so they are not available to react |
|
|
What does a catalyst do and how much of it is needed? |
- changes the rate of reaction and is unchanged at the end of the reaction - only a small quantity of catalyst is needed to catalyse a large mass of reactants; each catalyst is specific to a particluar reaction |
|
|
Where can the relative atomic mass of an element be found and how is it calculated? |
- can be found on the periodic table - atomic mass (A) is the largest number shown for each element; these numbers can be found above the atomic symbol in the table - the relative atomic masses must be added up in the correct order, if there are brackets in the formula, in order to arrive at the correct relative formula mass (M). |
|
|
How do you calculate the relative formula mass of calcium nitrate - Ca(NO3)2? |
- Ca = 40, N = 14 and O = 16 - work out masses inside the bracket - NO3 = 14+ 16 x 3 = 62 - multiply the total by the number outside the bracket - 62 x 2 = 124 - work out the remaining number - 40 = 40 - add the totals from steps 2 and 3 to find the relative formula mass - 124 + 40 = 164 - answer = 164 |
|
|
What is conservation of mass and why is mass conserved? |
- in any chemical equation, the total mass of the reactants equals the total mass of the products, which is called the conservation of mass - mass is conserved because atoms cannot be created nor destroyed, only arranged into different compounds - e.g. - KOH + HNO3 ---> KNO3 + H2O formula mass of reactants = 119 formula mass of products = 119 - the mass of the product is directly proportional to the mass of limiting reactant used. |
|
|
How do you calculate reacting masses (using the decomposition of copper carbonate)? |
- in a chemical symbol equation, there are the same number and type of atoms on each side of the equation - symbol equation for the decomposition of CuCO3 - CuCO3 ---> CuO + CO2 - relative atomic mass - Cu = 64, C = 12 and O = 16 - relative formula masses - 64 + 12 + (16 x 3) ---> (64 x 16) + 12 + (16 x 2) so... 124 ---> 80 + 44 so... 124g CuCO3 will give 80g of CuO or 12.4g CuCO3 will give 8.0g CuO |
|
|
How do you predict the yield of a reaction and how do you find the mass of CuCO3 needed to make 20g of CuO? |
- predictions can be made by using the symbol equation for a reaction - to find the mass of CuCO3 needed to make 20g of CuO: write a balanced symbol equation - CuCO3 ---> CuO + CO2 - find out atomic masses of the elements and then work out the relative formula mass - CuCO3 = 124 and CuO = 80 - add units -124g CuCO3 makes 80g of CuO - find the mass of CuCO3 needed to make 1g of CuO - 80g/124g CuCO3 makes 1g CuO - multiply to the amount of CuCO3 needed - 80g x 20g __________ = 12.9g 124g |
|
|
What cab relative formula mass calculations be used to find out, what is this usually in practice and what is the formula? |
- used to find out how much product should be made (the predicted yield) in a chemical reaction - in practice, the actual yield produced is less, due to losses on the practical methods used actual yield percentage yield = _____________ x 100 predicted yield |
|
|
Why do industrial processes need to have as high a percentage yield as possible? |
- so that they can reduce the amount of reactants wasted, which is wasteful and costly - so that they can reduce their costs by ensuring that enough reactants are used, as too little reduces the amount of product |
|
|
What is the formula for atom economy? |
Mass of desired products __________________________ x 100 = atom economy Sum of M of all products
- when calculating atom economy, use only the relative formula mass of the products |
|
|
How can the atom economy be found for complex balanced symbol equations? |
- using the same formula, but the relative formula masses first need to be calculated from the atomic mass data before completing the calculation |
|
|
Calculate the atom economy of when iron ore is reduced to iron (Fe2O3 + 3CO ---> 2Fe + 3CO2): |
- Fe = 56, O = 16 and C = 12 - CO2 is a waste product, so the atom economy of this process is calculated as follows: - work out the relative formula mass for each product: 2Fe = 56 + 56 = 112 3CO2 = 3(12 + 16 + 16) = 132 - atom economy = 112/144 x 100 = 45.9% |
|
|
Why does industry want as high an atom economy as possible? |
- to reduce the production of unwanted products that will need to be disposed of (which often adds to overall costs) - to make the process more sustainable by making better use of the reactants, so conserving raw materials and avoiding the need to get rid of wasted products. |
|
|
What types of processes are bond breaking and bond making and how do you decide which type a chemical reaction is? |
- bond breaking is an endothermic process - bond making is an exothermic process - the amount of energy made and produced needs to be compared - all chemical reactions involve bond making (exothermic) and bond breaking (endothermic) |
|
|
What can diagrams be used to represent (to do with making and breaking bonds)? |
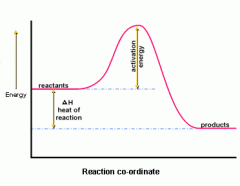
- diagrams can be used to represent bond energy changes and this diagram shows an overall exothermic reaction - in the first stage energy is needed to break reactants into separate atoms - in stage 2, atoms join to form new bonds, releasing energy - if more energy is released than needed, the reaction is exothermic - if more energy is needed than released, the reaction is endothermic |
|
|
How is the energy transferred by a fuel calculated? |
using this formula: m x c x triangle T = energy transferred (in J) where m = mass in water heated (in g) c = specific heat capacity of water (4.2 J/g degrees celsius) triangle T = temperature change (in degrees celsius) |
|
|
Describe the process needed to find out the energy released by 1g of solid fuel: |
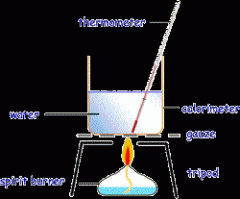
- measure out the mass of 1g of fuel - pour 100g of water into a copper calorimeter (1cm3 of water = 1g) - heat the water with the burning fuel - measure the temperature rise - repeat with different fuels to ensure fair testing e.g. the same distance from the flame to the calorimeter - ensure reliability by repeating results |
|
|
How can the mass of fuel burnt be found? |
- by measuring the mass of the burner and the fuel before and after heating - the formula energy = m x c x triangle T is used to calculate energy transferred - to calculate the energy in a gram of fuel the following formula is used: energy released (in J) _________________________ = energy per gram mass of fuel burnt (in g) |
|
|
How are chemicals needed in large quantities produced and what are the advantages of this process? |
- chemicals needed in large quantities, such as ammonia and sulfuric acid, are made using continuous processes Continuous Processing: - makes large amounts of the product 24 hours a day, seven days a week - takes place in large chemical plants with good transport links; plants are highly automated so have minimal labour costs, making the product cheaper - takes less energy to maintain - as long as the process can be kept running - than to stop and start the process |
|
|
How are chemicals needed in small quantites produced and what are the advantages of this process? |
- chemicals needed in small quantities, such as medicines, are made using batch processing Batch Processing: - makes a fixed amount - allows batches to be made and stored until needed - allows quantities to be made that can be sold within a given time as many drugs have a 'sell by' date - makes it easy to make a new batch when needed - makes it easy to change production to a different product |
|
|
What are the disadvantages of continuous processing? |
Although the advantages listed for continuous processing mean that the cost per tonne is very small, the disadvantages are that: - the process is very inefficient if not in constant use - there is a very high initial building and set-up cost for these chemical plants |
|
|
What are the disadvantages of batch processing? |
Batch processing has the advantage of flexibility, there are also a number of disadvantages with this method of production: - each batch has to be supervised so it is labour intensive and very costly - time is needed for cleaning if the product line is changed - it is inefficient as the production is not in use all the time |
|
|
Why are drug prices set to take account of the development costs? |
- it can take about 10 years to develop and test a new drug and each country has strict safety laws that pharmaceutical companies must follow - many compounds need to be made before one may be useful to develop - raw materials are often rare and costly - many raw materials are found in plants, and are difficult to extract |
|
|
What does extracting a raw chemical from a plant involve? |
- crushing to disrupt and break cell walls - boiling in a suitable solvent to dissolve the compounds - chromatography to separate and identify individual compounds - isolating, purifying and testing potentially useful compounds
|
|
|
What do pure chemicals have and what is thin layer chromatography used for? |
- definite melting and boiling points - used to test the purityo of a compound by comparing the speed of movement against a pure known sample |
|
|
Why is it difficult and costly to get a licence for a new drug? |
- thousands of compounds often need to be tested to find effective ones - likely compounds need to be tested on living tissue to ensure safety - long-term trials on humans are needed to identify possible side effects - many similar compounds need to be developed to try to reduce side effects - recommended doses need to be shown to be effective - the research needs to be independently verified - patents expire before costs are recouped and others can make a version |
|
|
What are allotropes and what are the allotropes of carbon? |
- different structures of the same element - diamond, graphite and fullerenes |
|
|
What are fullerenes and what do they do? |
- carbon structures that form spheres or tubes and can be used: - to carry and deliver drug molecules around the body - to trap dangerous substances in the body and remove them |
|
|
Describe the appearance and structure of Buckminsterfullerene: |
- contains 60 carbon atoms in a sphere - its formula is written C60 - each sphere is so small it is measured in nanometres - a nanometre is 10 to the -9 metres long |
|
|
What are both diamond and graphite? |
- giant covalent structures of carbon atoms - every carbon atom in diamond makes strong covalent bonds in different directions and in graphite the carbon atoms bond to make layers |
|
|
Describe the structures of diamond, graphite and a complete particle of buckminsterfullerene: |
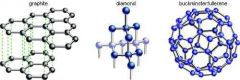
|
|
|
Why are diamonds useful? |
- it is the hardest natural substance known and has a very high melting point and boiling point - the imperfections that naturally occur in diamonds form cleaving plates which allow them to be shaped - these properties make them ideal for use in cutting tools, and as jewellery, as the facets reflect light |
|
|
Why is graphite useful? |
- it has a high melting and boiling point, but the layers can slide over each other - it is used in pencils, and as a high temperature lubricant |
|
|
Descrive the structure and bonding of diamonds and how its structure gives it specific properties: |
- giant covalent bonding involves electron sharing and every carbon atom in diamond is covalently bonded to four others in a three-dimensional tetrahedral lattice with all the outer shell electrons being shared the structure of diamond gives it specific properties: - strong covalent bonds in all directions make diamond hard - large amounts of energy are required to break the bonds, giving a high melting point of 3350 degrees celsius - an absence of free electrons, meaning diamond does not conduct electricity |
|
|
Descrive the structure and bonding of graphite and how its structure gives it specific properties: |
- every carbon atom in graphite is covalently bonded to three others in flat hexagonal layers - this formation leaves an unshared outer shell electron - this delocalised electron is free to move along the layer - the separate layers are only weakly attracted to each other this structure gives graphite specific properties: - the strong covalent bonds give graphite a melting point similar to diamond - the delocalised electrons make it a good electrical conductor - when force is applied, the weak forces between the layers slide over each other, this slippery nature makes it an ideal high-temperature lubricant |
|
|
What does giant covalent bonding form, what does this type of structure and bonding mean, and what are nanotubes? |
- compounds with millions of very strong fixed bonds - any compound with this structure will have a high melting point - if the bonds are formed in different directions, the substance is hard - if the bonds form in layers, it will be easy to cut in slices - if there are no free electrons, the substance will not conduct electricity - nanotubes can be used in catalyst systems - atoms of the catalyst can be attached to the large surface areas on the nanotubes |

