![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
10 Cards in this Set
- Front
- Back
|
Define a Covalent Bond
|
A shared pair of electrons by two atoms, each electron donated by either atom
Usually non-metal & non-metal And similar in high electronegativity |
|
|
Define an Ionic Bond
|
An atom transfers an electron to another atom causing opposite charges on them so they have a electrostatic attraction and are held together in a crystal lattice
Usually in metal & non-metal Large difference in electronegativity |
|
|
Define a mole
|
The amount of a substance that has the same number of particles as there are atoms in 12g of 12C
|
|
|
Define polar
|
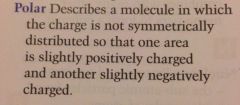
|
|
|
Define a polyatomic ion
|
A charged particle with two or more atoms joined by covalent bonds
|
|
|
Define a polar bond
|
A bonding pair of electrons that isn't shared equally
|
|
|
Define dipole
|
Opposite charges separated by a short distance in a molecule/ ion
|
|
|
Define relative molecular mass
|
The mean mass of a molecule compared to one-twelfth the mass of a 12c atom
|
|
|
Define First Ionisation Energy
|
Energy needed to remove one mole of electrons from one mole of gaseous atom
|
|
|
Simple molecular
|
Molecules attracted to each other by weak intermolecular forces
|

