![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
19 Cards in this Set
- Front
- Back
|
Q: In the periodic table, what is a group and what is a column? |
A: A group is Vertical and a column is Horizontal |
|
|
Q: When elements are heated what do they emit? |
A: A Coloured flame |
|
|
Q: What is the charge of a proton, electron and neutron? |
A: Proton is +1 Electron is -1 Neuron is 0 (neutral) |
|
|
Q: Atoms have the same number of _____ to _____? |
A: Same number of protons to electrons |
|
|
Q: How do you find the relative atomic mass? |
A: Number of protons + Number of Neutrons |
|
|
Q: What is the electron arrangement of Oxygen? |
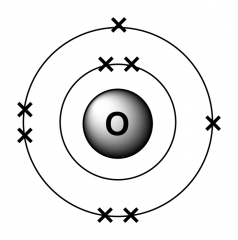
A: 2,8,7 |
|
|
Q: What is the correlation between electron shells and energy levels? |
A: The closer the electron shell to the nuclues the lower the energy level |
|
|
Q: How are elements arranged in the periodic table? |
A: In Proton Number |
|
|
Q:Are group 1 metals reactive? |
A: No they are Unreactive |
|
|
Q: The number of electrons in the outer shell of an atom is the same as what? |
A: its group number |
|
|
Q: What is special about group 1 metals?
|
A: you can cut them, they are soft |
|
|
Q: What do group 1 metals react with? |
A: Water, they fizz and produces hydrogen |
|
|
Q:What is the equation for water with group 1 elements? |
A: Water + Metal--> Metal Hydroxide + Hydrogen |
|
|
Q:What is the reaction of group 1 metals and chlorine? |
A: Creates a yellow flame and a white solid (sodium chloride) |
|
|
Q: What are elements in group 7 called? |
A: Halogens |
|
|
Q: What is bad about halogens? |
A: They are corrosive and toxic |
|
|
Q: What are ionic compounds? |
Q: Compounds in group 1 and 7 that have high melting points. They contain charged particles. |
|
|
Q: How are ions formed |
A: Losing or gaining 1 electron |
|
|
Q:What do ions have on thier outside |
A: A full outer shell |

