![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
136 Cards in this Set
- Front
- Back
|
What does the nucleus contain
|
Protons and neutrons
|
|
|
What surrounds the nucleus in shells
|
Electrons
|
|
|
What's the charge and mass of a proton
|
Positive, 1 a.m.u
|
|
|
What's the charge and mass of a neutron?
|
Neutral, 1 a.m.u
|
|
|
What's the charge and mass of an electron?
|
Negative and 1/1836 a.m.u
|
|
|
What's the atomic number?
|
Number of protons in the nucleus- also equivalent to number of electrons
|
|
|
What's the mass number?
|
Sum of protons and neutrons in the nucleus of an atom
|
|
|
What's an isotope?
|
Atoms of the same element with different numbers of neutrons but same protons (and electrons)
|
|
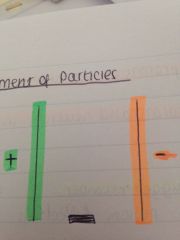
What is the movement of particles?
|
Neutrons pass through as they're neither attracted or repelled to either plate
Protons go to the negative plate as opposites attract and like charged repel Electrons go to the positive plate as opposites attract and like charges repel |
|
|
What is the mass spectrometer?
|
An instrument which can measure the masses and relative concentration of atoms and molecules
|
|
|
How many stages to the mass spectrometer are there and what are they?
|
5; vaporisation, ionisation, acceleration, deflection, detection.
|
|
|
What happens during vaporisation?
|
The sample is vaporised to allow free movement of particles (gaseous)
|
|
|
What occurs during ionisation?
|
An electron gun fires electrons at high speed/energy which knock into the gaseous atoms and cause an electron to be knocked out of the atom, forming a positive ion
|
|
|
What's the equation that can represent ionisation?
|
X (g) ------> X+(g) + e-
|
|
|
What are the particles accelerated using?
|
An electric field
|
|
|
What does the electric field consist of?
|
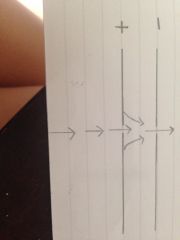
2 parallel plates with a hole in each, the first being positive the second negative.
|
|
|
What are the ions deflected using?
|
A magnetic field
|
|
|
What does the amount of deflection depend on?
|
-the mass of the particles ( lighter=more deflection)
-the charge of the particles (less charge=less deflection) |
|
|
What formulae can you used from using a mass spectrum output to calculate the ram of an element?
|
(M/z x abundance)+(M/z x abundance)+(M/z x abundance)
/ Total abundance |
|
|
When we talk about species what do we need to think about?
|
Element, charge, mass
|
|
|
What is formed if on occasion atoms lose two electrons in the mass spectrometer?
|
A 2+ ion to be formed
|
|
|
What will a peak look like when a 2+ ion is formed? And what will it's m/z value be?
|
It'll be small and the m/z value will be half the mass number if the isotope
|
|
|
What's relative molecular mass?
|
This is the average mass of a molecule compared with 1/12th of the mass of a carbon-12 atom
|
|
|
What causes a molecule to fragment and split up?
|
When they are ionised and lose an electron their bonds weaken
|
|
|
What causes lots of peaks on the spectrum?
|
Smaller ions that are produced
|
|
|
How do you know which is the RMM of a chemical on the spectrum?
|
It is that of highest value of m/z when none of the chemical has fragmented NOT THE MOST ABUNDANt
|
|
|
Are the shells close to the nucleus high or low in energy?
|
Low they increase as you get further away
|
|
|
What formulae can you used from using a mass spectrum output to calculate the ram of an element?
|
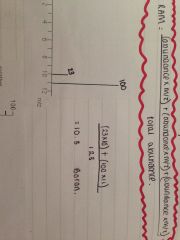
G
|
|
|
What does the amount of deflection depend on?
|
-the mass of the particles ( lighter=more deflection)
-the charge of the particles (less charge=less deflection) |
|
|
What is the second shell of an element divided into?
|
2 sub shells;
The 's' sub shell which can contain 2 electrons And the 'p' sub shell which can contain six |
|
|
What's the third shell of an element divided into?
|
3 sub shells - s,p and d which can hold 10 electrons
|
|
|
What is the order of filling the sub shells?
|
The lowest energy sub shell is filled first
|
|
|
Does this mean they go in number order?
|
No. The 4s sub shell is filled before the 3d sub shell even though it's slightly further from the nucleus it's lower in energy
|
|
|
What are sub shells always in order of?
|
Energy
|
|
|
What do the factors causing deflection combine to make?
|
A mass/charge ration ... (M/z)
|
|
|
What happens if ions collide with walls?
|
They'll become neutral and sucked out by a vacuum pump
|
|
|
What do the charged particles then do?
|
Hit the detector and cause a current which is amplified to create a spectrum
|
|
|
What is the broken down process of detection
|
An electron moves from the metal onto the ion making the ion neutral
A space is left amongst the electrons in the metal and electrons in wire move to fill it A flow of electrons in wire is detected as an electric current which can be amplified and recorded |
|
What's the third shell divided into?
|
3 sub shells - s,p and d which can hold 10 electrons
|
|
|
How can you allow different isotopes to hit the detector?
|
Alter the magnetic field
|
|
|
What's relative atomic mass
|
The average mass of an atom compared with 1/12th of the mass of a carbon-12 atom
|
|
|
What are sub shells always in order of?
|
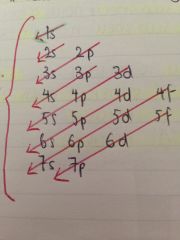
Energy
|
|
|
When filling the p and d sub shells how do the electrons go in and why?
|
Desperately because of repulsion
|
|
|
What happens to the copper to make it differ?
|
An electron is substituted from 4s to 3d to make the shell full and more stable
|
|
|
If the electron notation for beryllium is 1s^2 2s^2 what is the orbital notation?
|
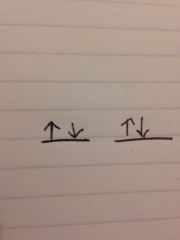
|
|
|
How are positive ions formed?
|
By the loss of electrons
|
|
|
What happens then to the electron arrangement of ions?
|
They gain or lose electrons accordingly
|
|
|
What happened to the electron arrangement of the ion O^2-
|
The 2p she instead of having 4 electrons gains to to make 6 electrons and this makes it stable
|
|
|
What happens to the electron arrangement of ions on the d block?and why?
|
Electrons are lost out of the 4s sub shell first- when the 3d sub shell is being filled the electrons repel and the 4s ones are further from the nucleus so they're at a higher energy and therefore are lost first
|
|
|
What's the definition of ionisation energy?
|
The amount of energy required to remove 1 mole of electrons from one mole of gaseous atoms to form 1 mole of gaseous positive ions
|
|
|
What's the simple ionisation energy definition?
|
How much energy is need to remove an electron
|
|
|
What are three key terms used (and their definitions) when talking about ionisation energies?
|
Distance- distance from nucleus and electron
Shielding- shielding from shells between the nucleus and outer shell electron Effective nuclear charge- the attraction of the positive nucleus on the outer shell electrons |
|
|
What happens to the ionisation energy down and group(with reason)?
|
It decreases due to increased distance and shielding weakening the attraction between the nucleus and the outer electron making it easier to lose an electron
|
|
|
What do they do after this?
|
They pair up
|
|
|
What generally happens to the ionisation energy across a period?(with reason)
|
It increases as the effective nuclear charge increases(more protons in nucleus) but shielding and distance stay the same as there is no change in shell amount making the attraction between the outer electrons stronger making it harder to remove an electron
|
|
|
What is the second ionisation energy?
|
The amount of energy required to remove one mole of electrons from one mole of gaseous positive ions to form one mole of gaseous do-positive ions
|
|
|
What do the second ionisation energy require lots more of and why?
|
Energy as it is harder to remove an electron from a positive ion because the attraction is greater. The second ionisation energy is always greater then the first
|
|
|
B
|
G
|
|
|
If the electron notation for beryllium is 1s^2 2s^2 what is the orbital notation?
|
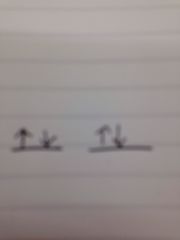
|
|
|
How many shells do you fill when filling orbitals?
|
One at a time
|
|
|
Why do the paired electrons go in opposite directions?
|
Because of repulsion
|
|
|
How're the sub shells firstly filled?
|
Will all the electrons moving in the same direction and then they're filled (like seats on a bus)
|
|
|
If you simplify in terms of argon how many electrons are represented for?
|
The first 18
|
|
|
How many exceptions are there?and of what elements?
|
2- copper and chromium
|
|
|
Why do their arrangements differ?
|
As their stability is associated with half-full and full sub shells
|
|
|
What happens in chromium to make it differ?
|
And electron from the 4s sub shell is substituted so that the orbital 3d shell is half full and more stable
|
|
|
What is an ionic bond?
|
The attraction between oppositely charged ions
|
|
|
What type of elements are the metallic structured ones?
|
Metals
|
|
|
What is the number of electron groups dependant on?
|
The number of electron groups around the central atom
|
|
|
How are linear shaped molecules formed? And what is the angle size
|
When two bond pairs of electrons repel equally as far apart as possible - 180
|
|
|
What is an example of a linear shaped molecule?
|
CO2
|
|
|
What is formed when 3 bond pairs of electrons repel equally as far apart as possible?
|
A trigonal planar shaped molecule
|
|
|
What's the bond angle for a trigonal planar shaped molecule and an example of one?
|
120 and
|
|
|
What is formed when 4 bond pairs mutually repel as far apart as possible?
|
A tetrahedral shaped molecule is formed with the bond angle 109.5
|
|
|
What is an example of a tetrahedral shaped molecule?
|
CH4
|
|
|
When is a coordinate bond formed?
|
When one atom donates and shares both electrons for the bond
|
|
|
What is an example of a coordinate bond and how is it shown on a stick diagram?
|
With and arrow and ammonia
|
|
|
What are ionic bonds known as?
|
Hard crystalline solids because they are strong and therefore have high melting points
|
|
|
What shape is formed when 5 bond pairs of electrons equally repel as far apart as possible?
|
A trigonal bi pyramidal shaped molecule
|
|
|
What is an example of a linear shaped molecule?
|
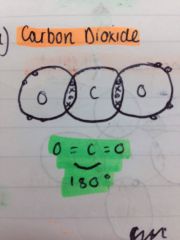
CO2
|
|
|
How is an octahedral shaped molecule formed ?
|
By mutual repulsion between 6-bond pairs of electrons equally repelling as far apart as possible
|
|
|
What's the bond angle for a trigonal planar shaped molecule and an example of one?
|

120 and
|
|
|
What is a pair of electrons unshared also known as?
|
A lone pair
|
|
|
What is an example of a tetrahedral shaped molecule?
|
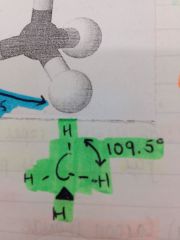
CH4
|
|
|
Why can't an ionic solid conduct?
|
In order to conduct you need freely moving ion ls but as a solid they are in a fixed position
|
|
|
What is an example of a coordinate bond and how is it shown on a stick diagram?
|
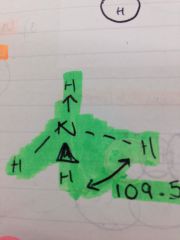
With and arrow and ammonia
|
|
|
What is a covalent bond?
|
The attraction between the nuclei and the shared electron pair
|
|
|
How would PCl5 be shown on a stick diagram?
|
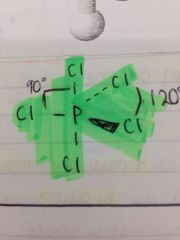
|
|
|
What type of elements are the monatomic structured ones?
|
Group 0
|
|
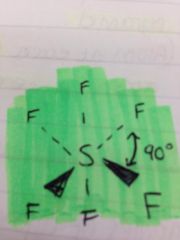
What is this an example of?
|
An octahedral shaped molecule
|
|
|
What type of elements are the giant covalent structured ones?
|
Si Graphite Diamond
|
|
|
What type of elements are the ionic structured ones?
|
Metal and non metal compounds
|
|
|
What is an ionic bond?
|
The attraction between oppositely charged ions
|
|
|
What type of elements are the metallic structured ones?
|
Metals
|
|
|
What is the number of electron groups dependant on?
|
The number of electron groups around the central atom
|
|
|
How are linear shaped molecules formed? And what is the angle size
|
When two bond pairs of electrons repel equally as far apart as possible - 180
|
|
|
What is an example of a linear shaped molecule?
|
CO2
|
|
|
What is formed when 3 bond pairs of electrons repel equally as far apart as possible?
|
A trigonal planar shaped molecule
|
|
|
What's the bond angle for a trigonal planar shaped molecule and an example of one?
|
120 and
|
|
|
What is formed when 4 bond pairs mutually repel as far apart as possible?
|
A tetrahedral shaped molecule is formed with the bond angle 109.5
|
|
|
What is an example of a tetrahedral shaped molecule?
|
CH4
|
|
|
When is a coordinate bond formed?
|
When one atom donates and shares both electrons for the bond
|
|
|
What is an example of a coordinate bond and how is it shown on a stick diagram?
|
With and arrow and ammonia
|
|
|
What are ionic bonds known as?
|
Hard crystalline solids because they are strong and therefore have high melting points
|
|
|
What shape is formed when 5 bond pairs of electrons equally repel as far apart as possible?
|
A trigonal bi pyramidal shaped molecule
|
|
|
What is an example of a linear shaped molecule?
|
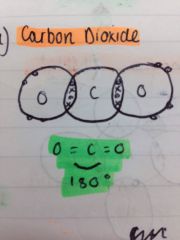
CO2
|
|
|
How is an octahedral shaped molecule formed ?
|
By mutual repulsion between 6-bond pairs of electrons equally repelling as far apart as possible
|
|
|
What's the bond angle for a trigonal planar shaped molecule and an example of one?
|

120 and
|
|
|
What is a pair of electrons unshared also known as?
|
A lone pair
|
|
|
What is an example of a tetrahedral shaped molecule?
|
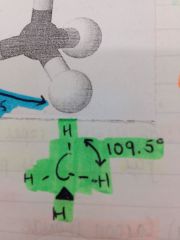
CH4
|
|
|
Why can't an ionic solid conduct?
|
In order to conduct you need freely moving ion ls but as a solid they are in a fixed position
|
|
|
What is an example of a coordinate bond and how is it shown on a stick diagram?
|
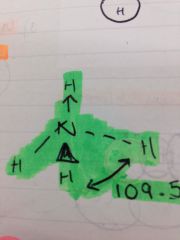
With and arrow and ammonia
|
|
|
What is a covalent bond?
|
The attraction between the nuclei and the shared electron pair
|
|
|
How would PCl5 be shown on a stick diagram?
|
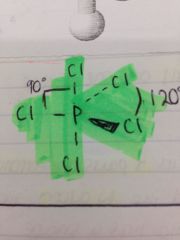
|
|
|
What type of elements are the monatomic structured ones?
|
Group 0
|
|
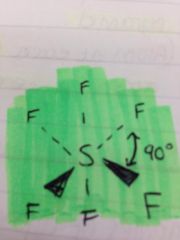
What is this an example of?
|
An octahedral shaped molecule
|
|
|
What type of elements are the giant covalent structured ones?
|
Si Graphite Diamond
|
|
|
What type of elements are the ionic structured ones?
|
Metal and non metal compounds
|
|
|
If for example ammonia has four bond pairs but one of these are lone how many arms does it have?
|
3
|
|
|
If the two atoms in a molecule are the same eg h2 what is formed? And how will electrons be attracted? What is this called?
|
A true covalent bond is formed and so atoms will attract the electrons equally - said to be a non polar bond
|
|
|
If there is no difference in electronegativity between molecules (identical) what is the bond?
|
Non-polar
|
|
|
If there is a small difference in electronegativity what is the bond?
|
Polar
|
|
|
If there is a difference in electronegativity where are the electrons pulled towards?
|
The most electronegative atom
|
|
|
What will the difference in electronegativity affect?
|
Types of forces between molecules in COVALENT STRUCTURE
|
|
|
What are intermolecular forces and what are they always?
|
Forces between molecules and they're always weak+in covalent structures
|
|
|
Three types of intermolecular forces in order of strength w-->s?
|
Van der Waals
Dipole-Dipoles Hydrogen bonds |
|
|
What's a dipole dependant on and where does it exists?
|
Difference in electronegativity and occurs in polar molecules
|
|
|
How do lone pairs differ to bonding pairs?
|
They're attracted ONLY by the centre element nucleus whereas shared pairs are attracted also to the other element there- so are therefore pulled closer to it
|
|
|
What is the difference between repulsion between a lone pair and a bonding pair? And what is the effect?
|
Lone pairs repel more then bonding pairs - and so for each lone pair the angle is reduced by2
|
|
|
What's a bent molecule?
|
When there is 2 lone pairs and 2 bonding pairs
|
|
|
When there are six pairs of electrons and 2 of which are lone pairs what position do they adapt?
|
One furthers apart from eachother
|
|
|
What's electronegativity?
|
The ability of an atom to attract an electron pair in a covalent bond
|
|
|
What three factors affect electronegativity?
|
Distance
Shielding Effective nuclear charge |
|
|
What happens to electronegativity down a group?
|
It decreases as there is increased shielding and distance which weakens the attraction
|
|
|
What happens to electronegativity across a period?
|
It increases as the effective nuclear charge increases but shielding and distance stay the same
|
|
|
What are the top five electronegative elements?
|
F
O N Cl Br |

