![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
73 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Atom |
The smallest particle of an element that retains the chemical properties of an element |
|
|
|
Compound |
A substance made of atoms of 2 or more different elements which are chemically bonded |
|
|
|
Which one is matter Air or Light |
Air |
|
|
|
What are the vertical columns on the periodic table called |
Groups |
|
|
|
What are the horizontal lines on a periodic table called |
Periods |
|
|
|
What is mass of a compound according to the law of conservation of mass |
Mass is all of the atoms in a compound |
Calculating molar mass CO C= 12.0107 + O= 15.9994; CO= 28.0101g/mole |
|
|
What was Rutherford's gold experiment and how did he conclude about empty space in atoms |
Rutherford's gold experiment test at a piece of gold between a particle emitter and ZnS to determine the amount of space that atoms occupy. Atoms have a nucleus. |
|
|
|
What are positively charged particles in an atom |
Protons |
|
|
|
What are the particles with no electric charge known as |
Neutrons |
|
|
|
Which particles have almost the same Mass among protons neutrons and electrons |
Protons and neutrons |
Electrons have virtually no Mass |
|
|
What does the nucleus of an atom contain |
Mass |
|
|
|
What makes an atom electrically neutral |
The equal number of protons and electrons make an atom electrically neutral |
|
|
|
Isotopes of the same element are atoms with different what |
Masses |
|
|
|
All atoms of oxygen contain how many protons |
8 |
|
|
|
The atomic number of an atom is equal to its number of what |
Protons |
|
|
|
Nitrogen-14 has a mass of how many Amu |
14 Amu |
Look at the mass following the element |
|
|
The neon isotope consists of 9 protons and 9 electrons and 10 neutrons its mass number is what |
Atomic mass number 19 |
Add the neutrons and the protons |
|
|
Sodium has an atomic number of 11 and a mass number of 23 it has how many protons how many electrons and how many neutrons |
11 protons 11 electrons and 12 neutrons |
Atomic number is equal to protons and electrons and atomic mass is equal to protons and neutrons |
|
|
Carbon 13 with an atomic number of 6 has how many neutrons |
Has 7 neutrons |
Protons plus neutrons equal the mass |
|
|
Helium-4 contains number of two neutrons, so how many protons does it contain |
Two proton |
Protons plus neutrons equals mass |
|
|
Gamma rays visible light in microwaves have the same |
Speed |
Speed of light |
|
|
Speed of light is equal to the product of what and what |
Wavelength and frequency |
Equation of light and electromagnetic spectrum |
|
|
Electromagnetic radiations have what kind of behavior |
Wave-like Behavior |
|
|
|
Going from ground state to excited state electrons do what with energy |
Absorb energy |
|
|
|
An electron cloud is the region outside the nucleus where what are most probably found |
Electrons |
|
|
|
Add ground state electron have what possible energy |
Low possible energy |
|
|
|
3s level is found in a what energy level than 2s level |
A higher energy level than 2s level |
|
|
|
What is the maximum number of electrons in sublevels S P D and F |
S equals 2. p equals 6. d equals 10. and f equals 14 |
|
|
|
What is the atomic number of beryllium with electron configuration of 1s2 2s 2 |
4 |
Two plus two equals 4 |
|
|
Write electron configuration for calcium and chlorine |
Configure atomic number |
|
|
|
Number of electrons in the highest energy level of xenon |
8 |
Look at the energy level then count all of the electrons in that level |
|
|
List all the noble gases |
Helium neon argon Krypton Xenon radon |
|
|
|
The periodic law states that the physical and chemical properties of elements are periodic functions of their atomic what |
Atomic numbers |
|
|
|
The periodic law allows some properties of an element to be predicted based on its what |
Position on the periodic table |
|
|
|
Elements in a group / column have similar what |
Chemical properties |
|
|
|
The atomic number of boron the first element in he is fine he atomic number of the second element in this group is what |
13 |
Used periodic table |
|
|
Chlorine atomic number 17 belongs to group 17 how many electrons does chlorine have in its outermost energy level |
7 |
|
|
|
In nature alkali metals occur as |
Compounds |
|
|
|
Most reactive group of nonmetals are |
Halogens |
|
|
|
Noble gases are what |
Highly unreactive |
|
|
|
Which group is more reactive alkali metals or alkaline earth metals |
Alkali metals |
|
|
|
What is the atomic radius |
The half of the distance between the nuclei bonded together |
|
|
|
Put these elements in increasing electronegativity O.N.I.F.C |
Electronegativity increases left to right |
Electronegativity increases left to right |
|
|
Atomic radii what's as atomic number increases |
Increases |
|
|
|
London dispersion forces are blank than dipole-dipole forces |
Weaker than dipole dipole |
London dispersion forces are the weakest |
|
|
London dispersion forces are stronger if electrons are blank in number and masses of molecules are blank |
London dispersion forces are stronger if electrons are increasing in number and masses of molecules are increasing |
|
|
|
What forces are attractions between positive and negative regions of molecules |
Dipole dipole forces are attractions between positive and negative regions of molecules |
|
|
|
Water has a higher boiling point because of what bonding between molecules |
Hydrogen bonding between molecules |
|
|
|
IBr has a higher boiling point than I2 because IBr is a ______ molecule and I2 is a ______ molecule |
Polar/ Non Polar |
|
|
|
Is gravitational force a type of intermolecular forces |
No |
Hydrogen bonding dipole dipole London dispersion and ionic bonding intermolecular forces |
|
|
Can particles in gases in liquids change position with other particles |
Yes |
Particles move more freely in gases and liquids |
|
|
Put these in increasing order of strength in terms of intermolecular forces |
Gas liquid solid |
|
|
|
In what state are particles closer liquid or gas |
Liquid |
Pop in the freezer |
|
|
What is evaporation and sublimation |
Evaporation is liquid to gas and sublimation is solid to gas |
|
|
|
When a liquid changes into a solid energy particles do what |
Decrease |
|
|
|
In which state do particles hold each other strongly |
Solid |
Freezing water to ice |
|
|
Solids have definite shape and volume because particles do what with their positions |
They cannot change their positions |
|
|
|
Solids have what shape or volume |
Solids have definite shape and volume |
|
|
|
Liquids have what shape or volume |
Liquids have no definite shape but definite volume |
|
|
|
Gases have what shape or volume |
Gases have no definite shape and no definite volume |
|
|
|
What do the two and four in helium 4 with a atomic number 2 represent |
2 and 4 represent atomic number and atomic mass |
|
|
|
What do the 101 and 256 in Mendel veium 256 with an atomic number of 101 represent |
Atomic number and atomic mass |
|
|
|
What does the 238 in uranium 238 represent |
Atomic mass |
|
|
|
In a nuclear reaction unstable nuclei change the number of protons and neutrons to become what and produce large amounts of what |
To become stable and to produce large amounts of energy |
|
|
|
Balance the following equation beryllium plus helium equals carbon plus what |
Neutron |
First find what it equals then match it with its symbol |
|
|
Balance the following equation neptunium equals plutonium plus what |
Electron capture |
Find what equals then find the symbol to match |
|
|
During radioactive decay the nucleus disintegrates into a what and what nucleus |
Into a lighter and stable nucleus |
|
|
|
Write the symbol for alpha particle |
Helium 4 atomic number 2 |
|
|
|
What is the symbol for beta particle |
Beta 0 atomic number -1 |
|
|
|
Gamma rays are what kind of radiation |
Electromagnetic radiation |
|
|
|
What is the half life of an isotope if 125 grams of a 500 gram sample of the isotope remain after 3 years |
1.5 half life |

|
|
|
What is the half life of an isotope of 50 grams of a 100 gram sample of the isotope remain after 5 years |
Five half-life |
Refer to number 72 |
|
|
Example of balancing an equation |
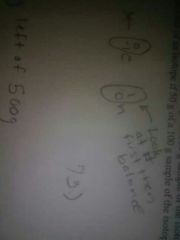
|
|

