![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
49 Cards in this Set
- Front
- Back
|
What is the main purpose of TCA cycle? Other purposes? |
main: capture high energy electrons via redox of carbon compounds and transfer to FADH2 and NADH
other: some ATP made, lipids from acetyl-CoA
|
|
|
where in the body is there a lot of mitochondria? why? where not so much? |
A lot in the heart, because needs a lot of energy to pump
not many in liver because not an actively proliferating tissue |
|
|
what is located in the inner mitochondrial membrane? |
oxphos proteins and enzymes for ETC |
|
|
what is in the matrix? |
TCA cycle |
|
|
what is in the cristae? |
cofactors/proteins involved in TCA and ETC |
|
|
PDH links.... |
glycolysis to TCA |
|
|
how does pyruvate enter mitochondria? what happens if it does or doesnt? |
enters via active transport to become acetyl-CoA or OAA
if doesn't enter becomes lactate |
|
|
which step in PDH mechanism would be good for pharmacological intervention? |
the regeneration of lipoamide because it is a key reaction necessary to allow the pathway to continue |
|
|
how is the entry of pyruvate into TCA regulated (list) |
- energy need - covalent modifications of PDH - enzymatic activity (transcription, translation) |
|
|
high energy charge regulates PDH via.. |
products (ATP, Acetyl CoA, NADH) turn down activity of PDH |
|
|
low energy charge |
ADP and pyruvate stimulate PDH activity |
|
|
how does structural changes in citrate synthetase allow its reaction to occur correctly? |
OAA binds first to 2 binding sites on CS, then acetyl coA can bind
confirmation closes and condensation to citrate occurs, avoids indiscriminate hydrolysis of acetyl-coA |
|
|
which citric acid cycle intermediate may be used as biosynthetic precursors? |
citrate is the main one exported, in the cytosol an enzyme changes it back to Acetyl-CoA to make lipids |
|
|
in a rapidly proliferating cell, how is the slowdown of TCA prevented when citrate is being used to make lipids? |
Glutamine-->glutamate-->a-KG which enters TCA |
|
|
Mutant vs wt IDH |
wt: citrate-->a-KG
mutated: a-KG--> D-2-HG which isnt normally found in the body (biomarker) |
|
|
NADP+--> NADPH in mutant vs wt IDH |
wt: IDH creates lots of NADPH mutant: almost no NADPH present because it's being used to produce NADP+ and is consuming a lot |
|
|
how does mutated IDH affect transcription? |
When genes are not being transcribed are methylated at glycine residues
2-HG blocks enzymes that strip glycines off (using a-KG) from transcribing genes at that loci |
|
|
what is an inhibitor of aconitase and how does it work? |
fluoroacetate-->fluoroacetyl-Coa + citrate--> fluorocitrate which is an ireversible inhibitor of aconitase that reduces TCA and respiration |
|
|
why must biosynthetic precursors like lipids be made de novo? |
have specifications that must be met depending on where they are in the cell |
|
|
which of the TCA enzymes are in the inner mitochondrial membrane rather than the matrix? |
SDH (forms complex with FADH2) |
|
|
how is malate-->OAA driven forwards despite not having favourable free energy? |
by products (OAA, NADH) |
|
|
how do tumors rely on glycolysis when they shut off PDH? |
when oxygen decreases in the tumor, transcription factor HIF-1 gets PDK-1 to hydrolyze ATP and adds Pi to PDH to turn it off
use Glycolysis for ATP
do pathway in cytosol (glutamine--> glutamate--> a-KG--> isocitrate cleaved by ACL-->citrate--> AcCoA and some OAA) |
|
|
why must TCA and ETC occur in certain locations |
- build gradient - keeps everything together to react correctly in right order |
|
|
why not have all ETC energy released at once? |
less control, which you want so the energy is used specifically to make ATP |
|
|
experiment: measure electron transfer potential |
set up two half reactions with the reference reaction having an electron transport potential of zero
the reduction potential of sample is observed voltage at the start of the experiment |
|
|
how are ROS formed? |
incomplete reduction of O2 |
|
|
ROS are good for...? |
Pathogen clearance (anti-viral response) Activation of T cells proliferation ROS can kill cells (radiotherapy) Inactivates Fe-S clusters (i.e. aconitase |
|
|
ROS are bad for..? |
Oxidative damage: lipids DNA proteins Aging, cancer (mutations) |
|
|
ROS detox |
superoxide dismutase: converts ROS to O2 and peroxide
peroxide is still reactive though so catalase breaks it down to water and oxygen |
|
|
how does cytochrome C link bioenergetics to cell fate? |
usually found only in mitochondria but leaks out when apoptosis signals received
form apoptosome complex to initiate caspases which initiate cascade towards apoptosis |
|
|
What happens when you take away a cell's mitochondrial DNA? |
Rho0 cells formed that have no functional respiratory chain
don't die and can proliferate
make fatty acids through other pathways in cytosol |
|
|
what are the two main components of ATP synthase? where are they located? which does catalysis? |
F1: matrix (catalysis)
F0: mitochondial membrane |
|
|
structure of ATP synthase : F1 |
5 different polypeptide chains, alpha and beta chains in a hexameric ring
stalk contains gamma and epsilon proteins in chains |
|
|
structure of ATP synthase : F0 |
beta parts can have open, loose, or tight states each allowing whether nucleotides can go in or out or whether they're converted to ATP |
|
|
open state |
binds and releases nucleotides |
|
|
loose state |
binds ADP and Pi but not release them |
|
|
tight state |
binds ATP and can convert ADP + Pi to ATP, but can't release the ATP that's formed |
|
|
binding and transformation of ADP+ Pi to ATP can occur.... |
in the absence of proton motor force |
|
|
what powers ATP synthesis? how? |
- proton flow through F0 C-ring - has two half channels, one in cytosol and one in matrix -Aspartic residue captures proton as gradient builds up and H+ enters cytoplasmic half - ring rotates in a clockwise direction and proton is shifted to the matrix channel where it's released |
|
|
rotation of c ring is coupled to |
ATP synthesis |
|
|
Why must there be mechanisms to interchange NAD+ and NADH in the cell? |
not directly interchangeable |
|
|
how many ATP from one Glc? |
about 30 |
|
|
how are defects in mitochondria measured? |
oxygen levels |
|
|
how does FCCP affect mitochondria? |
proton ionophore that causes protons to leak into matrix (collapses proton gradient) |
|
|
how does oligomycin affect mitochondria? |
blocks ATP synthase (dramatic O2 conjucation decrease) |
|
|
Rot/Myx vs oligo vs FCCP effect on ETC graph |
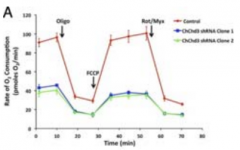
Oligo mycin decrease O2 conjugatoin, then FCC collapses gradient so conj. goes back up to re-establish gradient, Rot/Myx decreases again |
|
|
what do uncoupling proteins do? where are they? |
dissipate proton gradient, stop ATP production
found on the membrane, especially in fatty acid tissues |
|
|
why do proliferating cells prefer glycolysis over oxphos? |
make more than you need |
|
|
what can be made from glucose from oxphos |
36 ATP |

