![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
3 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
Chemical reaction 3 |
1 synthetic reaction 2. decomposition reaction 3 single replacement reaction 4. Double displacement reaction |
Synthetic reaction Or composition reaction.
|
|
|
When two substances combine to form third substance it is called synthetic reaction. Single reactant breaks down in to elements of simple compounds it is known as a decomposition reaction |
Atoms of One element replace atom of another element in a compound is called single replacement reaction. Two compounds react to form two new compounds it is known as double replacement reaction. When two compounds react to form another new two compounds is known as double replacement Reaction. |
|
|
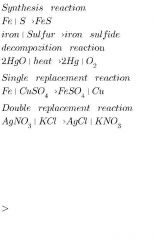
All reactions example
|

2 .Decomposition reactions example |

3.Singhal replacement reaction and 4.double replacement reaction examples. |

