![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
7 Cards in this Set
- Front
- Back
|
Energy cannot be created or destroyed. This means that both sides of an equation have to be balanced(have the same amount of atoms on both sides) |
combustion of hydrogen: 2H2+ O2 = 2H2O |
|
|
relative atomic masses = Neutron = 1 proton = 1 electron = 0 |
relative atomic charges = neutron = 0 proton = +1 electron = -1 |
|
|
Periodic table Group = electrons in outer shell of the atom Period = number of shells in the atom |
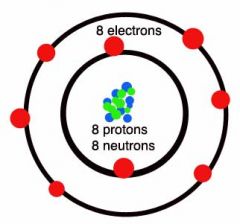
Oxygen Group 6 Period 2 |
|
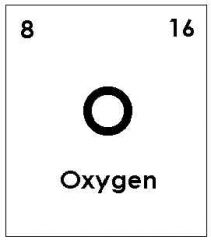
left = Proton number (atomic number) right = atomic mass (number of sub-atomic particles in the nucleus) |
There are the same number of protons and electrons in an atom The number of electrons in the shells always stick to the pattern 2,8,8,2. E.g. oxygen has 8 electrons so it has 2 in the first shell and 6 in the second |
|
|
Relative atomic mass = ((mass of isotope 1 x % of isotope 1) x ( mass of isotope 2 x % of isotope 2)) --------------------------------------------------------------------- 100% |
Isotope = form of an element with the same atomic number but different atomic mass. Ion = a more stable form of an atom where electrons are added or taken away in order to make the fullest possible outer shell. as a result, the charge of the atom will change. |
|
|
Filtration = paper used to catch all of the unwanted substance but lets the liquid (filtrate)through Chromatography = water running over something to separate it into its base components Distillation = evaporating one thing (liquid) out of another by heating. can be used to purify water. |
mixture = when compounds or elements intermingle but don't react together compound = a substance made when two or more elements are chemically bonded together |
|
|
|
|

