![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
26 Cards in this Set
- Front
- Back
- 3rd side (hint)
|
What is the ideal gas law equation? |
PV=nRT |
Pressure and Volume relate to what? |
|
|
What are the seven strong acids? |
HCIO4 (perchloric acid), HI (Hydroiodic acid), HBr (Hydrobromic acid), HCl (Hydrochloric acid), H2SO4 (sulfuric acid), HNO3 (Nitric acid), H2NO3 (Nitric acid) |
5 acids have 1 leading H, 2 have 2. |
|
|
What are the 8 strong bases? |
LiOH, NaOH, KOH, RbOH, CsOH, Ca (OH)2, Sr (OH)2, Ba (OH)2 |
5 have 1 trailing OH, 3 have 2. |
|
|
S = ? ... Relating entropy to microstates |
K ln W |
|
|
|
G = ? Definition of Gibbs free energy |
H - TS |
|
|
|
ΔG = ? Free energy change at constant temperature |
ΔH - TΔS |
|
|
|
What is the equation for the determination of joules (J) at a given atomic energy level? |
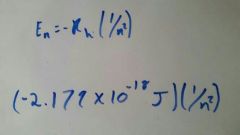
|
|
|
|
What is the equation for the change in energy (J) of an electron after a change in energy level? |
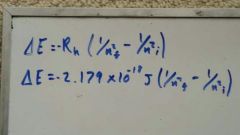
|
|
|
|
Definition of effective nuclear charge. |
Z eff = Z - σ |
|
|
|
What is the definition of density? |
D=m/v |
|
|
|
How is atomic mass defined? |
Number of protons + number of neutrons. |
|
|
|
What is the equation for percent composition of an element in a compound? |
(n x molar massof element / molar mass of compound) * 100 |
|
|
|
% yield = ? |
(Actual yield / theoretical yield) * 100 |
|
|
|
How is molarity defined? |
Moles of solute / liters of solution |
|
|
|
What is the equation for the dilution of solution? |
M1V1 = M2V2 |
|
|
|
What is general boyles law? |
P1V1 = P2V2 |
Relates pressure and volume. |
|
|
What is general Charles' law, (volume) |
V1/T1 = V2/T2 |
Relates volume and temperature. |
|
|
What is Charles' law, (pressure) |
P1/V1 = P2V2 |
Relates pressure and temperature. |
|
|
What is the equation for Avogadro's law? |
V = k4n |
Relates volume to a constant |
|
|
Equation used for calculating changes in pressure, volume, temperature or amount of gas present. Any pair of variables can be removed and the equation remains useful. |
PV/nT = PV/nT (first side is sub 1 second side is sub 2) |
|
|
|
Equation used for calculating density and molar mass. |
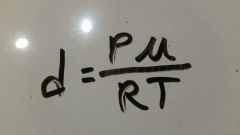
|
|
|
|
Equation used to calculate the root mean square speed of gas molecules. |

|
|
|
|
What is the equation for Graham's law of diffusion and effusion. |
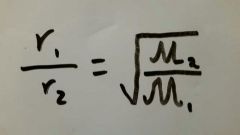
|
|
|
|
van der Waals equation modifications of the ideal gas equation for non ideal gasses. |
(P + an^2/V^2)(V - nb) = nRT |
|
|
|
Equation relating speed of a wave, wavelength of a wave, and the frequency of a wave. |
υ = λν |
|
|
|
Equation for the relationship between standard free energy change and the equilibrium constant. |
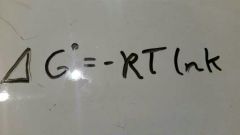
|
|

