![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
49 Cards in this Set
- Front
- Back
|
What are the 2 ways of classifying alcohols? |
Aliphatic and aromatic Primary,Secondary and tertiary |
|
|
What does Aliphatic mean in terms of alcohols? |
general formula CnH2n+1OH - provided there are no rings
• the OH replaces an H in a basic hydrocarbon skeleton |
|
|
What does Aromatic mean in terms of alcohols? |
in aromatic alcohols (or phenols) the OH is attached directly to the ring•
an OH on a side chain of a ring behaves as a typical aliphatic alcohol |
|
|
How do you identify a primary, secondary or tertiary alcohol from it's displayed formula? |
on a primary alcohol, the carbon which carries the hydroxyl group (-OH) is only attached to ONE alkyl group.
In secondary alcohols the carbon carrying the OH-group is attached to TWO alkyl groups and in tertiary alcohols to THREE. |
|
|
What are the physical properties of alcohols? |
Hydrogen bonding- make boiling points higher-more energy required to break the intermolecular bonds…..
Soluble because- OH groups form hydrogen bonds with the water so the molecule dissolves
|
|
|
Why do longer chain alcohols get harder to dissolve? |
Larger chains are less soluble because as the molecule gets longer, the C chains don’t form hydrogen bonds as they’re non-polar so are hydrophobic and so the OH group dissolves but the longer chain can’t…Polar and Non polar mixture-suspension droplets of nonpolar enclosed by polar
|
|
|
What is elimination, what reagent does it use and what are it's conditions? |
Sometimes called dehydration-opposite of making alcohols
Reagent-conc H2So4 180 degress (high temp) excess acid makes an alkene and H20 |
|
|
What is a substitution reaction? what does it use? |
Reaction with strong acid catalyst H+ (e.g. conc H2SO4) and halide ions X- form a salt (e.g.NaX)
or Strong acids such as HCl (catalyst H+ and halide ion X-) |
|
|
How is oxygen used to differentiate between primary,secondary and tertiary alcohols? what reagent is used? |
The usual reagent is acidified potassium dichromate(VI)
orange colour to green Primary Easily oxidised to aldehydes and then to carboxylic acids. Secondary Easily oxidised to ketones Tertiary Not oxidised under normal conditions. |
|
|
How are Aldehydes recognised? |
Aldehydes recognised by CHO group at end of carbon chain as it has a hydrogen at the end of the group it can be oxidised further into a carboxylic acid.
|
|
|
give the practical details of distilling primary alcohols? |
the acidified K2Cr2O7 is dripped into the warm alcohol
aldehydes have low boiling points - no hydrogen bonding - they distil off immediately if it didn’t distil off it would be oxidised to the equivalent carboxylic acid to oxidise an alcohol straight to the acid, reflux the mixture |
|
|
How do you show oxidation in a balenced equation? |
[o] |
|
|
explain the oxidation of Secondary alcohols? |
Secondary alcohols are easily oxidised to ketones
The alcohol is refluxed with acidified K2Cr2O7. (under normal conditions) However, on prolonged treatment with a powerful oxidising agent they can be further oxidised to a mixture of acids with fewer carbon atoms than the original alcohol. |
|
|
Explain the oxidation of tertiary alcohols? |
Tertiary alcohols are resistant to normal oxidation
|
|
|
Explain esterification |
Alcohols can react with carboxylic acids to form esters in a cindensation reaction
water is removed and a carbon- oxygen single bond forms. |
|
|
How do you name an ester? |
When naming an Ester- Alcohol first (methyl) then carboxylic acid- carboxylic ending is -oate.
|
|
|
What is the ending of Aldehydes and keytones |
-al keytone is -one as they are carbonyls |
|
|
How do you name an alcohol? |
CnH2n+1OH suffix -ol is added to the end, you have to indicate which carbon the alcohol group is attached to if there are 2 OH groups the molecule is a -diolo, and if there are three it is a -triol. |
|
|
What is a halogenoalkane? |
Halogenoalkanes are compounds in which one or more hydrogen atoms in an alkane have been replaced by halogen atoms
|
|
|
What are the physcial properties of halogenoalkanes and why? |
immiscible with water-Won’t dissolve because doesn’t make hydrogen bonds- even though it is polar- generally forms a layer on top
•C-Hal bond is polar• the bigger the halogen atom /the larger the number of halogen atoms the higher the boiling point. - more electrons- more induced dipole dipole- more halogens- more perm dipole dipole from stronger polarity •larger halogen atoms (Br or Cl) cause greater environmental damage than smaller halogen atoms (F); this is important when designing replacements for CFCs. |
|
|
what are the chemical properties of halogenoalkanes? |
Carbon –halogen (C─Hal) bonds can break either homolytically or heterolytically• Homolytic Fission forms radicals eg when a halogenoalkane absorbs radiation of the right frequency
carbon-halogen bonds more polar and has a lower bond enthalpy so is more likely to be more attacked by radicals |
|
|
What is nucleophillic substitution and why does it occur in haloalkanes? |
Heterolytic fission is more common in lab conditions using polar solvents such as ethanol or ethanol and water. The polar C-Hal bond can break, leaving a negative halide ion and positive carbocation.
Negatively charged substances (or substances containing a lone pair) may react with the positive carbocation causing a substitution reaction |
|
|
Show nucleophillic substitution in bromoethene with an hydroxide ion |
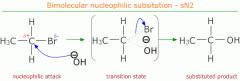
δ+ carbon is electron deficient and can be attacked by nucleophiles such as CN-, OH- and NH3 etc.
|
|
|
shoe nucleophillic substitution in ammonia |
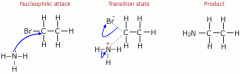
Whatever you do, you get a mixture of all of the products (including both amines and their salts.
|
|
|
How do you hydrolise an Haloalkane and what does it make? |
an alcohol hydrolysis with an hot aqueous alkali- gives an OH- ion so willl be standard nucleophillic substitution RX+ OH- --> ROH + X- Hydrolysis with water- water is a weak nucleophile but will eventually substitute for the halogen although this is a slower process than using a warm aqueous alkali RX + H20 --> ROH + H+ + X- |
|
|
Explain the bond enthalpy and hydrolysis reactions of haloalkanes as you go down the halogen group |
The stronger the C-Hal bond is, the harder it is to break. so The higher the bond enthalpy, the stronger the bond.•The bond enthalpies are stronger higher up the group, as the halogens are smaller.
•As you move down the group seven elements, the C-Hal bond gets weaker, thus the compounds get more reactive. |
|
|
explain why chloro compounds are bad for the eviromnment explain why bromo and ido compounds are used as intermediates in organic synthesis? |
•Chloro compounds are un-reactive and so stay in the environment for a long time → CFC’s can last long enough to make it into the stratosphere, where they catalyse the destruction of the ozone layer.
•Bromo and Iodo compounds are fairly reactive, which makes them useful as intermediates in organic compound synthesis. |
|
|
how do yuo measre hydrolysis rates? which should be fastest and why? |
set up flasks with the same amount of haloalkane on it add ethanol and warm it add warm silver nitrate put flask over a cross on the desk and time how long it takes for silver halide precipate to appear and for the cross to be obscured iodalkanes shuld react the fastest. and it decreases as you go up the group. fluroalkanes don't react at all. |
|
|
how do you identify unknown haloalkanes |
add a bit to a test tube with water, silver nitrate and ethanol and look at the precipitate it forms. |
|
|
What are CFC's, what are they used in and why? |
Chlorofluorocarbons CFCs are stable, volatile, non-flammable and non-toxicWere used in fridges, aerosol cans , dry-cleaning and air-conditioningHarmless in our atmosphere but dangerous when in the stratosphere, where the ozone layer can be found, which acts as a chemical sunscreen absorbing harmful UV radiation before it reaches the Earth.
|
|
|
How is Ozone formed? |
Ozone is constantly formed and depleted naturally in the ozone cycle:
O2(g) + hν → O(g) + O(g) O(g) + O2(g) → O3(g) O3(g) + hν → O2(g) + O(g) O(g) + O3(g) → 2O2(g) |
|
|
Why are CFC's bad for the Ozone layer? is it the same with Nitrogen oxide radicals? |
Free radicals formed from homolytic fission of CFCs destroy the ozone layer and catalyse the breakdown of thousands of ozone molecules (on average, 1 chlorine radical catalyses the breakdown of around 10000 ozone molecules before it forms a stable compound)
Initiation: CF2Cl2(g) + hν → CF2Cl●(g) + Cl●(g )Propagation 1: Cl●(g) + O3(g) → O2(g) + ClO●(g) Propagation 2: ClO●(g) + O(g) → O2(g) + Cl●(g) Overall: O3(g) + O(g) → 2O2(g) O2 cannot protect us from UV radiation A similar reaction takes place when free radicals from nitrogen oxides (NO●) reach the stratosphere.
|
|
|
What are the replacement for CFC's? |
Hydrochlorofluorocarbons, HCFCs- These have a shorter life in the atmosphere than CFCs, and much of them is destroyed in the low atmosphere and so doesn't reach the ozone layer. HCFC-22 has only about one-twentieth of the effect on the ozone layer as a typical CFC.
Hydrofluorocarbons, HFCs- Because these HCFCs don't contain any chlorine, they have zero effect on the ozone layer. HFC-134a is now widely used in refrigerants, for blowing foamed plastics and as a propellant in aerosols. Hydrocarbons-Again, these have no effect on the ozone layer, but they do have a down-side. They are highly flammable and are involved in environmental problems such as the formation of photochemical smog. Nowdays, most aerosols are pump-spray systems or nitrogen propelled. industrial fridges and freezers use ammonia as a coolant gas and carbon dioxide to make expanded polymers these substances do have drawbacks but are currently the least enviromentally damaging alternatives. the ozone holes are shrinking.
|
|
|
What is the greenhouse effect? what are the main green house gases? and how do they contribute to global warming? what are the factors that contribute to the amount of greenhouse effect a gas has? |
When gases in the trosophere absorb some of the infrared radiation from the sun and re-emits it back in all directions, including earth- this keeps us warm. Water vapour, Carbon dioxide and Methane the C=O and O-H and C-H bonds absorb IR raditation (infra-red) making the bonds in the molecule vibrate more. this extra energy is passed to other molecules by collsions in the air and gives that molecule kintetic energy. raising the overall temperature. How much IR one molecule of the gas can absorb How much of that gas there is in the atmosphere How long the gas stays in the atmosphere for. |
|
|
What is global warming? What evidence is there for global warming? |
human activities over the last 50 years (after the industrial revolution) has caused greenhouse gas concentrations to skyrocket which enhances the greenhouse effect so more heat is being trapped and so the earth is getting warmer. by analysing seawater,air and ice samples scientists can see a direct correlation between Earth's temperature and CO2 emissions However, correlation doesn't mean causation so scientists have found a mechanism that suggests strongly one causes the other so there is now a consensus among climate scientists the recent warming is anthropogenic (caused by humans) |
|
|
What are the consequences for global warming? |
Warmer oceans will expand, Ice sheets melt- sea levels rise- flooding- destruction of habitata and coral reefs that will be too deep to photosynthesis less predictable weather, droughts, storms, crop failiure and famine waterborn diseases etc. |
|
|
What efforts are going into preventing global warming? |
Kyoto protocol 1997- Industrialised countries had to reduce greenhouse emissions to agreed levels- this agreement came to an end in 2012 with no replacement though many governments by around 50% by 2050 UK- government created policies to try to use more renewable energy resources e.g. solar and wind farms reducing carbon dioxide emissions from coal-fired power stations the Government have also encouraged energy-efficient alternatives such as electric cars or energy-saving light bulbs |
|
|
Describe the polarity of alcohols |
Alcohols contain an Oh group the oxygen is much more electronegative than the hydrogen resulting in dipoles |
|
|
Describe and explain the solubility of alcohols in water |
Alcohol groups form hydrogen bonds with water and the compund dissolves hydrocrabon chains are non-polar and cannot form bonds with water hydrocarbon chain disrupts the bonding between other water molecules so the longer the chain the less soluble it is. |
|
|
Describe and explain the volatility of alcohols |
Alcohols can form H bonds with eachother giving them much lower volatility than other alkanes. the longer the chain the less volatile due to increased van der waals |
|
|
What is volality |
how easy a substance can turn into a gas. the less volatile the higher the boiling point. |
|
|
What is the general formula for Halogenoalkane? |
CnH2n+1X (where X is the halogen) |
|
|
How are halogenoalkanes classified? |
like alcohols, primary secondary, tertiary |
|
|
State the tye of reaction hydrolysis of a halogenoalkane is |
substitution |
|
|
Define nucleophile |
an electron pair donor |
|
|
Describe the hydrolysis of a halogenoalkanw with a hot aqueous alkali |
heat and reflux needed |
|
|
Describe the rates of hydrolysis and explain them. |
Hydrolysis becomes easier as you go down the group because the bond enthalpies decrease significantly so the bonds are more easily broken and the weaker the bond the faster it will hydrolise C-F will notudergo nucleophillic substitution as the bond is too strong. |
|
|
What are the factors that favor elimination reactions? |
higher temperaturesa concentrated solution of sodium or potassium hydroxidepure ethanol as the solvent Tertiary/secondary haloalkanes |
|
|
What are the factors that favour substitution reactions? |
lower temperaturesmore dilute solutions of sodium or potassium hydroxidemore water in the solvent mixture Primary haloalkanes favour substitution reactions |

