![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
9 Cards in this Set
- Front
- Back
|
alcohols:
|
compounds whose molecules have a hydroxyl group (OH) attached to a saturated carbon.
|
|
|
ethers:
|
O atom is bonded to 2 C atoms
|
|
|
nomenclature of alcohols:
|
1. longest chain to which the OH is attached gives us the base name
- ending is ol. 2. # the longest chain from the end that gives the C bearing hydroxyl group the lowest # notes: OH has precedence over double and triple bonds common names for alcohols is alkyl alcohol such as methyl alcohol... etc. |
|
|
Nomenclature for ethers:
|
1. ethers are frequently given common functional class names
--- listed in alphabetical order both groups that are attached to the O atom and adds the word ether at the end (ex. ethyl methyl ether) 2. IUPAC: ethers are named as alkoxyalkanes, alkoxyalkenes and alkoxyarenes. The RO- group is the alkoxy group (ex:20methoxypentane) 3. cyclic ethers: - use replacement nomenclature in which we relate the cyclic ether to the corresponding hydrocarbon ring system and use the prefix oxa- to indicate that an O atom replaces a CH2 group (ex: oxacyclopropane) |
|
|
are ethers soluble in water?
|
yes due to H-bonding
|
|
|
as alcohols increase in size do they become less or more soluble in water?
|
less due to the hydrocarbon portion of the molecule length, long chain alcohols are more "alkane-like".
|
|
|
acid-catalyzed hydration of alkenes:
|
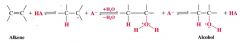
- markovnikov regioselectivity
- reaction is reversible - has limited synthetic utility b/c the carbocation intermidate may rearrange if a more stable or isoenergetic carbocation is possible by hydride or alkanide migration. |
|
|
oxymercuration-demercuration:
|
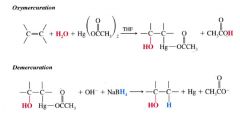
- alkenes react w/ mercuric acetate in a mixture of water and tetrahydrofuran (THF) to produce (hydroxyalkyl)mercury compounds, which can be reduced to alcohols w/ sodium borohydride and water
- markovnikov regioselectivity - generally takes place w/o the complication of rearrangements - is not stereoselective b/c even though the oxymercuration step occurs w/ anti-addition, the demercuration step is not stereoselective (mixture produced) |
|
|
hydroboration- oxidation:
|
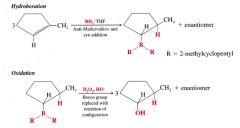
1. alkene reacts w/ BH3: THF or dibroane to produce an alkylborane.
2. oxidation and hydrolysis of the alkylborane w/ hydrogen peroxide and base yield an alcohol 3. B and H undergo syn addition to the alkene, treatment w/ hydrogen peroxide and base replaces the B w/ OH w/ retention of config. net addition of H and OH occurs w/ anti-markovnikov regioselectivity and syn steroselectivity |

