![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
55 Cards in this Set
- Front
- Back
|
Alkyl halides:
|
- halogen atom is attached to an sp3 hybridized carbon
- carbon arrangement therefore general tetrahedral - carbon-halogen bond... is polarized b/c halogen atoms are more electronegative to carbon. - halogen size increases as you go down the periodic table - C--X bond length increases and bond strength decreases as you go down periodic table |
|
|
Table representing carbon-Halogen bond length and bond strengths:
|
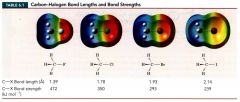
|
|
|
What are alkyl halides used for?
|
they are used as solvents for relatively nonpolar compounds and are used as the starting materials for the synthesis of many compounds
|
|
|
Vinylic halides:
|
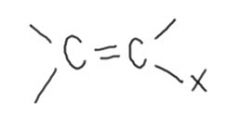
generally refers to a compound in which a halogen is attached to a C that also forms a double bond to another C.
|
|
|
Phenyl halide:
|
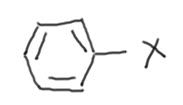
compounds in which halogen is attached to a benzene ring.
|
|
|
are most alkyl and aryl halides soluble in water?
|
they have very low solubilities to water.
|
|
|
Nucelophilic substitution reaction:
|
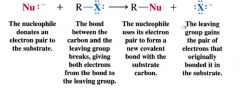
|
|
|
Nucleophile:
|
- love the (+) part of an atom
- when reacts w/ an alkyl halid... (+) part (center) that the nucleophile seeks is the c-atom that bears the x atom - any (-) ion/ any neutral molecule that has @ least one unshared e- pair. - reacts w/ an alkyl halid (substrate) by replacing the halogen substituent. |
|
|
what is the leaving group in a nucleophilic substitution reaction?
|
the halogen substituent... departs as a halide ion.
|
|
|
general reaction for nucleophilic substitution of an alkyl halide by water:
|
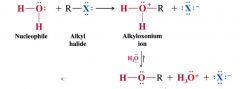
|
|
|
What makes a good leaving group?
|
- substituent that can leave as a relatively stable, weakly basic molecule/ anion.
- halogen atom of an alkyl halide is a good leaving group b/c once departed it is a weak base and stable anion. |
|
|
kinetics:
|
the rate of a reaction.
|
|
|
determing the rate of the reaction:
|
- reaction rates are known to be temp. dependent.
- can be determined experimentally be measuring the rate at which a compound disappears from the solution or the rate at which an ion or compound appears. |
|
|
Bimolecular:
|
two species are involved in the step whose rate is being measured.
|
|
|
molecularity:
|
# of species involved in a reaction step.
|
|
|
what causes an Sn2 reaction?
|
- nucelophilic
- substitution - bimolecular |
|
|
a schematic representation of orbitals involved in Sn2 reaction:
|
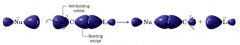
|
|
|
Describe an Sn2 reaction:
|
- nucleophile approaches the carbon bearing the leaving group from the backside (the side directly opposite the leaving group)
--- orbital that contains e- pair of the nucleophile (higest occupied molecular orbital, HOMO) begins to overlap with an empty orbital (lowest unoccupied molecular orbital, LOMO) of the carbon atom bearing the leaving group. -- as the reaction progresses.... bond between C & Nu strengthens... bond between C & LG weakens. - C-atom has it config. turned inside out (inversion) and LG is pushed away. |
|
|
how many steps are in an Sn2 reaction?
|
only one step, no intermediates
|
|
|
transition state:
|
- the formation of an unstable arragnement of atoms which proceeds the reaction
- fleeting arrangment of the atoms in which th enucleophile & the leaving group are both partially bonded to the carbon atom undergoing substitution - extremely brief existance |
|
|
concerted reaction:
|
bond formation & breaking simultaneously in a single transition
|
|
|
Exergonic:
|
a reaction that proceeds w/ negative free-energy
|
|
|
endergonic:
|
a reaction tat proceeds w/ a positive free-energy.
|
|
|
a mechanism for Sn2 reaction:
|
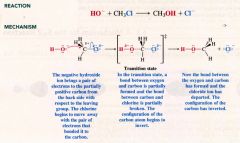
|
|
|
Free-energy diagram:
|
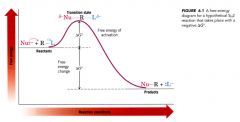
- a quantity that measures the process of the reaction
>> represents the changes in bond orders and bond distances that must take place as the reactants are converted to products. |
|
|
Free energy of activation (delta G):
|
- height of energy barrier above the level of reactants.
- the difference in free energy between the reactants and the transition state. |
|
|
Relationship between the rate of a reaction & the magnitude of the free energy of activation:
|

|
|
|
Inversion:
|
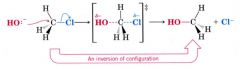
caused by a Sn2 backside attach.
- turns inside out in much the same way as an umbrella. |
|
|
The stereochemistry of an Sn2 reaction:
|
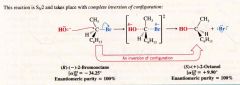
|
|
|
What is required for an Sn1 reaciton?
|
- substitution
- nucleophilic - unimolecular |
|
|
rate-determining step:
|
(rate-limiting step) slow step of the reaction.
|
|
|
How fast is the step of the formation of a carbocation in an Sn1 reaction?
|
carbocation formation, in general... takes place slowly b/c it is usually a highly endothermic process & is uphill in terms of free energy
|
|
|
mechanism for Sn1 reaction:
|
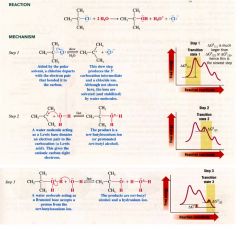
|
|
|
Describe the process of an Sn1 reaction:
|
step 1:
requries heterolytic cleavage of the C-X bond >> high endothermic >>high free energy of activation - departure of the halide takes place at all is largely b/c of the ionizing ability of the solvent (water) >> water molecules surround & stabilize the cation & anion that are produced step 2: intermediate cation reacts w/ water rapidly to produce ion. Step 3: rapidly transfers a proton to a molecule of water producing an alcohol |
|
|
Carbocation:
|
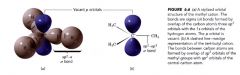
- trigonal planar
- sp2 hybridization - center C is electron deficient >> only has 6e- in its outer most shell ------ used to form sigma covalent bonds w/ H ------ p orbitals contain no e-s |
|
|
Carbocation:
|
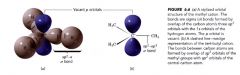
- trigonal planar
- sp2 hybridization - center C is electron deficient >> only has 6e- in its outer most shell ------ used to form sigma covalent bonds w/ H ------ p orbitals contain no e-s |
|
|
the relative stability of carbocations:
|
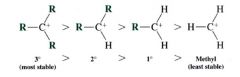
|
|
|
the relative stability of carbocations:
|
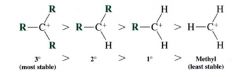
|
|
|
What influences the stability of carbocations?
|
hyperconjugation
|
|
|
What is hyperconjugation?
|
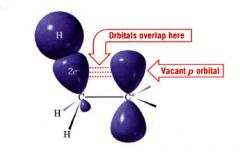
it involves e- delocalization (partial orbital overlap) from a filled bonding orbital to an adjacent unfilled orbital.
- unfilled orbital is the vacant p orbital of the carboncation & the filled orbitals are C-H and C-C sigma bonds at the carbons adjacent to the p orbital of the carbocation. - sharing of e- delocalizes the (+) charge which causes the molecule to stabilize better. |
|
|
the stereochemistry of Sn1 reactions:
|
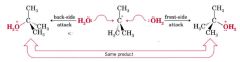
|
|
|
complete racemization:
|
if the original compound loses all of its optical activity in the course of the reaction
|
|
|
complete racemization:
|
if the original compound loses all of its optical activity in the course of the reaction
|
|
|
partial racemization:
|
if the original compound loses only part of its optical activity.
|
|
|
racemization:
|
takes place whenever the reaction causes chiral molecules to be converted to an achiral intermediate.
|
|
|
Another diagram of the stereochemistry of an Sn1 reaction:
|
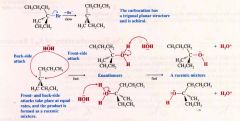
|
|
|
solvolysis reaction:
|
- an Sn1 reaction
- a nucleophilic substitution in which the nucleophile is a molecule of the solvent. (solvent & lysis: cleavage by the solvent) |
|
|
how solvolysis reaction occurs:
|
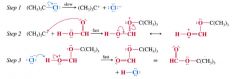
|
|
|
how solvolysis reaction occurs:
|
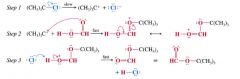
|
|
|
Important factors that affect the relative rate of Sn1 & Sn2 reactions:
|
1. the structure of the substrate
2. the concentration & reactivity of the nucleophile (bimolecular reactions only) 3. effect of the solvent 4. nature of the leaving group |
|
|
reactivity in Sn2 reactions:
|
methyl>primary>secondary>(tertiary-unreactive)
important factor is steric effects. |
|
|
Steric effects:
|
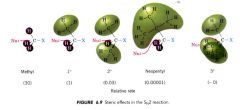
an effect on relative rates caused by the space-filling properties of those parts of a molecule attached at or near the reacting site.
|
|
|
Steric effects:
|
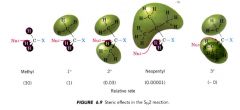
an effect on relative rates caused by the space-filling properties of those parts of a molecule attached at or near the reacting site.
|
|
|
Steric hindrance:
|
- one kind of steric effect
- the spatial arrangement of the atoms/ groups @ or near the reacting site of a molecule hinders/ retards a reaction. |
|
|
Why is it important to form a stable carbocation in an Sn1 reaction?
|
b/c it means that the free-energy of activaiton for the slow step of the reaction will be low enough for the reaction to take place @ a reasonable rate (only tertiary halids react by Sn1 mechanism most of the time)
|

