![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
68 Cards in this Set
- Front
- Back
|
Organic Chemistry:
|
The chemistry of the compounds of carbon.
|
|
|
Structural Theory:
|
1) atoms in organic compounds can form a fixed # of bonds using their outermost shell (valence e-s)
2) Carbon atom can use one/more of its valance e-s to form bonds to other carbon atoms |
|
|
Isomers:
|
different compounds that have the same molecular formula
|
|
|
Constitutional isomers:
|
different compounds that have the same molecular formula but differ in their connectivity, that is, in the sequence in which their atoms are bonded together.
--- usually have diff. physical properties --- diff. chemical properties |
|
|
Ionic Bonds:
|
(electrovalent) bond formed by the transfer of one or more e-s from one atom to another to create ions
|
|
|
covalent bonds:
|
bonds that result when atoms share e-s
--- when two/more atoms of the same/similar electronegativities react --- complete transfer of e-s doesn't occur --- products of covalent bonds are called molecules. |
|
|
Octect rule:
|
tendency for an atom to achieve a configuration where its valance shell contains 8 e-s
|
|
|
Ions:
|
atoms that gain/lose e-s and form charged particles
|
|
|
ionic bonds:
|
an attractive force between oppositely charged ions.
|
|
|
Electronegativity:
|

a measure of the ability of an atom to attract e-s
--- increases as we go across a horizontal row of the periodic table from left to right & as we go up a vertical column. |
|
|
Why do the ions form?
|

based on the lewis-kossel theory:
both atoms achieve the electronic configuration of a noble gas by becoming ions |
|
|
What are some properties of ions?
|
b/c of their strong electrostatic forces:
- usually very high melting solids - in a polar solvent the ions are solvated which create solutions that usually conduct an electric current. |
|
|
Salts:
|
ionic compounds
form only when atoms of very different electronegativitites transfer e-s to become ions |
|
|
what charge does a cation have?
|
+
|
|
|
what charge does an anion have?
|
-
|
|
|
Valence e-s:
|
e-s of an atom's outermost shell
|
|
|
What are the simple rules that allow us to draw a proper Lewis structure?
|
1. show the connections between atoms in a molecule or ion using only the valence e-s of the atoms involved.
2. for main group elements, the # of valence e-s a neutral atom brings to a Lewis structure is the same as its group # in the periodic table. 3. if the structure we are drawing is a negative ion (anion), we add one electron for each negative charge to the original count of valance e-s. If the structure is a positive ion (cation), we subtract one electron for each positive charge. 4. we try to give each atom the e- config. of a noble gas. draw structures where atoms share e-s to form covalent bonds or transfer e-s to form ions. ---- to achieve an octet of valence e-s, elements such as nitrogen, oxygen, and the halogens typically share only some of their valence e-s through covalent bonding. |
|
|
Why do atoms share e-s?
|
- to obtain the config. of an inert gas
- sharing e-s produces increased e- density between to positive nuclei ---- results: attractive forces of nuclei for e-s is the "glue" that holds the atoms together. |
|
|
Calculating formal charge:
|

|
|
|
A summary of Formal Charges:
|
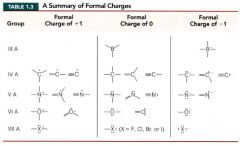
|
|
|
What are 2 important features of resonance structures:
|
- each atom has the noble gas configuration
- we can convert one structure into any other by changing only the position of the e-s or pi bonds |
|
|
Resonance Theory:
|
whenever a molecule or ion can be represented by two or more lewis structures that differ only in the positions of the e-s
1. none of the structures (resonance structures/resonance contributors) will be a correct representation for the molecule or ion. 2. The actual molecule/ion will be better represented by a hybrid (avg) of the structures. |
|
|
A representation of CO3^2- w/ its hybrid representation:
|
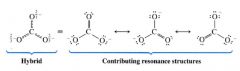
|
|
|
Electrostatic potential map:
|
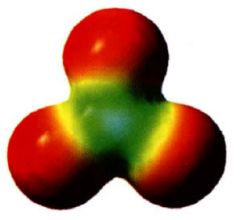
- shows e- density
- regions of relativity more (-) charge are red - more (+) regions are indicated by colors trending toward blue. |
|
|
Summary of rules for resonance:
|

|
|
|
Wave Functions:
|

- most often denoted by the Greek letter psi
- each corresponds to a different energy state for the e- ---- each state is a sublevel where one/two e-'s can reside |
|
|
Orbitals:
|
is a region of space where the probability of finding an e- is large
|
|
|
Aufbau Principle:
|
orbitals are filled so that those of lowest energy are filled first
|
|
|
Aufbau Principle:
|
orbitals are filled so that those of lowest energy are filled first
|
|
|
Aufbau Principle:
|
orbitals are filled so that those of lowest energy are filled first
|
|
|
Pauli exclusion principle:
|
a max. of two e-s may be placed in each orbital but only when the spins of the e-s are paired.
- an e- is permitted only one or the other of just tow possible spin orientations either up or down. |
|
|
Hund's Rule:
|
- when we come to orbitals of equal energy (degenerate orbitals) such as the 3p orbitals, we add one e- to each with their spins unpaired until each of the degenerate orbitals cotain one e-, then we begin to add a second e- to each degenerate orbital so that the spins are paired.
|
|
|
example of an e- configuration:
|

|
|
|
Heisenberg Uncertainty Principle:
|
We cant know simultaneously the position & momentum of an e-
--- we can't pin the e-s down as precisely. |
|
|
Molecular Orbitals (MOs):
|
- encompass both nuclei, &, in them, the e-s can move about both nuclei
- e-s are not restricted to the vicinity of one nucleus or the other as they were in the separate atomic orbitals. - may contain a max of two spin-paired e-s. |
|
|
Bonding molecular orbital:
|
- contains both e-s in the lowest energy state (ground state), of a hydrogen molecule
- formed when the atomic orbitals combine in the way by addition.... atomic orbitals of the same phase sign overlap ---- leads to reinforcement of the wave function in the region between the 2 nuceli there the value of psi^2 is larger - increasing the e- probability density in exactly the right place (region of space between nuclei) |
|
|
describe "the glue that holds the atoms together":
|
when e- density is large, the attractive force of the nuclei for the e-s more than offsets the repulsive force acting between the two nuclei.
|
|
|
Antibonding molecular orbital:
|
- contains no e-s in the ground state of the molecule
- formed by interaction of orbitals w/ opposite phase signs. - wave functions interfere w/ each other in the region between the two nuclei & a node is produced ---- @ node: psi = 0, therefore in the region between the nuclei psi^2, is also small therefore, if e-s were to occupy the antibonding orbital, e-s would avoid the region between the nuclei ---- only small attractive force of the nuclei for the e-s ---- repulsive forces (between the 2 nuclei & between the two e-s) would be greater than the attractive forces. |
|
|
Energy diagram for the molecular orbitals of hydrogen molecule:
|

- e-s are placed in molecular orbitals in the same way that they were in atomic orbitals
- 2 e-s (opposite spins) occupy the bonding molecular orbitals ---- total energy is less than in the separate atomic orbital "lowest electronic state/ground state" - an e- may occupy the antibonding orbital in what is called an excited state for the molecule.... ---- forms when the molecule in the ground state absorbs a photon of light of proper energy. |
|
|
Orbital hybridization:
|
a mathematical approach that involves the combining of individual waves functions for s & p orbitals to obtain wave functions for new orbitals
---- new orbitals have, in varying proportions.... the properties of the original orbitals taken separately. |
|
|
sp^3 orbitals:
|
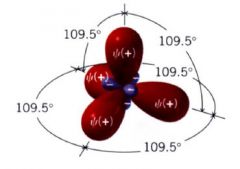
have one part the character of an s orbital and 3 parts the character of a p orbital.
|
|
|
Why do carbon and hydrogen share a strong bond?
|
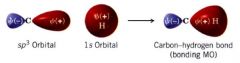
b/c sp^3 orbital has the character of a p orbital, (+) lobe of the sp^3 orbital is large.... extends relatively far from the carbon nucleus.
- (+) lobe of the sp^3 orbital that overlaps w/ positive 1s orbital of H to form the bonding molecular orbital of C-H bond ---- b/c the (+) lobe of the sp^3 orbital is large & is extended into space, the overlap between it & the 1s orbital of H is also large therefore, C-H bond is quite strong |
|
|
sigma bond:
|
bonds in which orbital overlap gives a bond that is circularly symmetrical in cross section when viewed along the bond axis....
--- all purely single bonds are sigma bonds. |
|
|
Alkanes:
|

single bonds
|
|
|
Alkenes:
|
double bonds
|
|
|
sp^2 Orbitals:
|
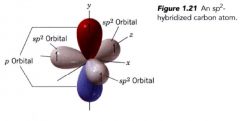
2 s orbitals is mathematically mixed w/ 2 of hte 2p orbitals (hybridized procedure applies only to the orbitals)
|
|
|
showing the process for obtaining sp^2 hybridized carbon atoms:
|
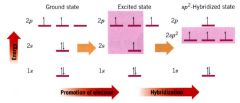
|
|
|
Pi bonds:
|
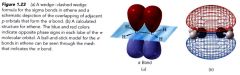
the sideways overlap of the p orbitals resulting in a new type of covalent bond
---- the parallel p orbitals overlap above & below the plane of the sigma framework has a nodal plane passing through the two bonded nuclei & between the pi molecular orbital lobes. |
|
|
alkynes:
|

triple bonds
|
|
|
sp orbitals:
|
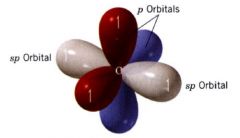
the 2 s orbitals & one 2p orbital of carbon are hybridized to form two sp orbitals.
|
|
|
energy diagram showing a process for obtaining sp-hybridized carbon atoms:
|
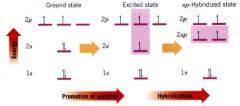
|
|
|
list bond length from shortest to longest of alkynes, alkenes, and alkanes.
|

|
|
|
VValence shell e- repulsion (VSEPR) model:
|
1. consider molecules (or ions) in which the central atom is covalently bonded to two or more atoms or groups
2. consider all the valence e- pairs of the central atom- both those that are shared in covalent bonds [called bonding pairs] and those that are unshared [nonbonding pairs] 3. b/c e- pairs repel each other, the e- pairs of the valence shell tend to stay as far apart as possible. The repulsion between nonbonding pairs is generally greater than that between bonding pairs 4. the geometry of the molecule by considering all of the e- pairs, bonding and nonbonding, describe the shape of the molecule or ion by referring to the positions of the nuclei. |
|
|
tetrahedral:
|

|
|
|
Trigonal Pyramid:
|

|
|
|
Angular / bent
|

|
|
|
Tigonal Planar:
|
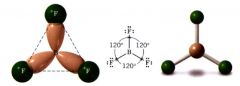
|
|
|
Linear:
|
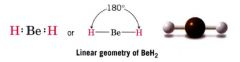
|
|
|
CHEAT SHEET: the hybridization of an atom:
|
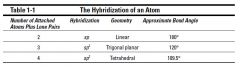
|
|
|
CHEAT SHEET: charge pattern for common atoms:
|
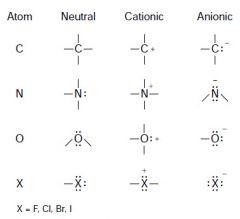
|
|
|
CHEAT SHEET: Basic steps to follow for drawing Lewis structures:
|

|
|
|
CHEAT SHEET: Determining whether a bond is ionic or covalent:
|
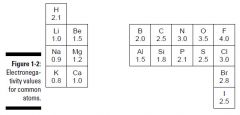
- if the electronegativity diff. between two atoms is 0.0, bond is purely covalent
- if the electronegativity diff. is between 0.00-2.0, the bond is considered polar covalent - if the electronegativity diff. is greater than 2.0, the bond is considered ionic |
|
|
CHEAT SHEET: Determine the dipole moment of a molecule:
|
1. draw the bond dipole vector for each of the bonds in the molecule
2. add the individual bond dipole vectors using mathematical vector addition to obtain the molecule's overall dipole moment. |
|
|
CHEAT SHEET: Drawing an orbital diagram:
|
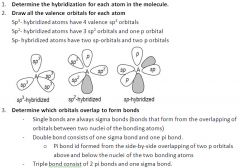
|
|
|
Another formula for formal charge:
|
= atoms of valence electrons - dots - sticks
|
|
|
CHEAT SHEET: rules for resonance structures
|

|
|
|
CHEAT SHEET: Rules for creating resonance structures:
|

|
|
|
CHEAT SHEET: How to determine stability in resonance structures:
|
1. resonance structures w/ fewer charges are more stable than resonance structures w/ more charges
2. for those molecules that are charged, the most stable resonance structures are the ones that place the charge on the best atom 3. resonance structures hat have all atoms w/ complete octets of e-s are more stable than resonance structures that have atoms with incomplete octets 4. the desire of a molecule to have all atoms with complete octets trumps the desire to put the charge on the best atoms. |

