![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
12 Cards in this Set
- Front
- Back
|
Where is solubility used?
Hint: Three basic techniques |
Crystallization, extraction and chromatography
|
|
|
How can solubility behavior be described?
|
Soluble, insoluble, g/L, mg/mL
Miscible (two liquids completely soluble in the other) Immiscible (do not mix and form two layers) |
|
|
Like dissolves like
|
Polar dissolves polar (dipole-dipole interaction)
Ion dissolves ion (ion-ion, ion-dipole) Nonpolar dissolves nonpolar (london/dispersion forces) |
|

|

|
|
|
Stronger the intermolecular forces
|
The higher melting/boiling points
|
|
|
Greater the surface area (less branch),
The heavier, |
Greater the boiling point
Hexane > Pentane |
|
|
Order them from weakest to strongest force
H-bond (NH or OH), ion-ioin, London/dispersion, ion-dipole, dipole-dipole |
London/dispersion, dipole-dipole, H-bond, ion-dipole, ion-ion
|
|
|
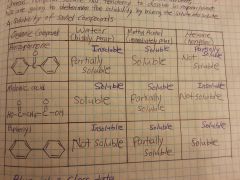
Would benzoic acid dissolve in water? NaOH?
|
No in water, although benzoic acid has a polar hydroxyl group, majority of the compound is nonpolar (aromatic ring = very nonpolar)
Yes in NaOH, due to acid - base interaction |
|
|
Would methanol dissolve in polar compounds? nonpolar compounds?
|
Yes in polar compounds due to OH, hydroxyl group.
Sometimes yes in nonpolar compounds because methanol also has a nonpolar CH3 part. |
|
|
Benzoic acid soluble in water? in NaOH? in HCl?
|
Not soluble in water because it has a very nonpolar phenol group (HOWEVER it becomes water soluble after addition of OH-)
Yes, soluble in NaOH No, not soluble in HCl |
|
|
Ethyl-4-aminobenzoate soluble in water? in NaOH? in HCl?
|
Not soluble in water because it has a very nonpolar phenol group (HOWEVER it becomes water soluble after addition of H+)
No, not soluble in NaOH Yes, soluble in HCl |
|
|
|

