![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
36 Cards in this Set
- Front
- Back
|
Chemical Bonds:
|
result of interactions between the charged particles-- electrons and protons--- that compose atoms.
|
|
|
Ionic bonds:
|
occur between metals and nonmentals-- involve the transfer of e-s from one atom to another
|
|
|
The formation of an ionic compound:
|
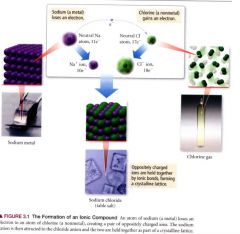
|
|
|
Covalent bonds:
|
occur between two or more nonmetals-- involve the sharing of e-s between two atoms.
- the shared electrons interact with the nuclei of both atoms, lowering their potential energy through electrostatic interactions with the nuclei. |
|
|
Chemical formula:
|
- indicates the elements present in the compound and the relative # of atoms or ions of each.
- the formula contains the symbol for each element and a subscript indicating the relative # of atoms of the element. - normally list the more metallic (or more (+) charged) elements first, followed by the less metallic (or more (-) charged) element. |
|
|
Empirical Formula:
|
simply gives the relative # of atoms of each element in a compound
|
|
|
Molecular formula:
|
gives the actual # of atoms of each element in a molecule of a compound
|
|
|
Structural Formula:
|
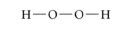
uses lines to represent the covalent bonds, shows how atoms in a molecule are connected or bonded to each other.
|
|
|
Molecular models:
|
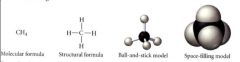
|
|
|
table representing molecular models of Benzene, Acetylene, Glucose, and Ammonia:
|
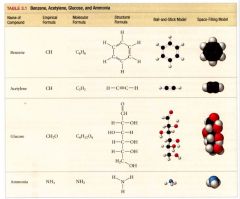
|
|
|
atomic elements:
|
those that exist in nature with single atoms as their basic units.
|
|
|
A molecular view of elements and compounds:
|
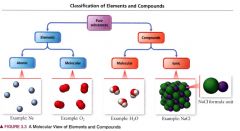
|
|
|
Molecular Elements:
|

do not normally exist in nature with single atoms as their basic units. Instead, exist as molecules, two or more atoms of the element bonded together.
- most exists as diatomic molecules - a few exists as polyatomic molecules. |
|
|
Molecular compounds:
|
usually composed of two or more covalently bonded nonmetals.
|
|
|
Ionic compounds:
|
composed of cations and anions bound together by ionic bonds
|
|
|
formula unit:
|
the smallest, electrically neutral collection of ions.
|
|
|
polyatomic ions:
|
an ion composed of two or ore atoms
ex: ClO- |
|
|
summary for writing formulas for ionic compounds:
|
- ionic compounds always contain positive and negative ions
- in a chemical formula, the sum of the charges of the positive ions (cations) must always equal the sum of the charges of the negative ions (anions). - the formula reflects the smallest whole-number ratio of ions. |
|
|
Procedure for writing formulas for ionic compounds:
|

|
|
|
naming an ionic compound:
|
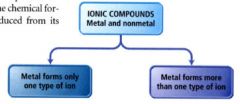
- identify it as an ionic compound (usually formed between nonmetals and metals)
- can be divided into two types depending on the metal in the compound. |
|
|
Binary Compounds:
|

are those containing only two different elements
|
|
|
some common anions:
|

|
|
|
naming binary ionic compounds containing a metal that forms more than one kind of cation:
|

|
|
|
some metals that form cations with different charges:
|
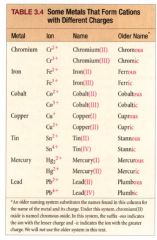
|
|
|
some common polyatomic ions:
|

|
|
|
how ionic compounds are named if there are only 2 ions in the series:
|
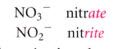
|
|
|
how ionic compounds are named if there are more than two ions in the series:
|

|
|
|
hypo =
|
less than
|
|
|
per=
|
more than
|
|
|
hydrate:
|

compound containing a specific number of water molecules associated with each formula unit.
|
|
|
Binary molecular compounds have the following form:
|

|
|
|
Acids:
|
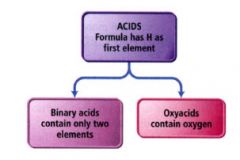
- molecular compounds that release hydrogen ions (H+) when dissolved in water.
- composed of hydrogen, usually written first in their formula, and one/more nonmetals written second. - are characterized by their sour taste and their ability to dissolve many metals. |
|
|
aqueous (aq) =
|
dissolved in water
|
|
|
Binary acids:
|

are composed of hydrogen and a nonmetal.
the names for binary acids have this form. |
|
|
oxyacids:
|

contain hydrogen and an oxyanion
- simply a combination of one or more H+ ions with an oxyanion. ex: HNO3 (aq) = nitric acid |
|
|
Formula mass:
|
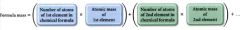
the average mass of a molecule
|

