![]()
![]()
![]()
Use LEFT and RIGHT arrow keys to navigate between flashcards;
Use UP and DOWN arrow keys to flip the card;
H to show hint;
A reads text to speech;
30 Cards in this Set
- Front
- Back
|
Alkane →(-H)→ ______
Alkane minus one hydrogen equals.... |
Alker Group
eg. methane →(-H)→ methyl |
|
|
What feature does an alkene have?
|
A carbon-carbon double bond
C=C |
|
|
What feature does an alkyne have?
|
A carbon-carbon triple bond
C≣C |
|
|
What kind of feature(s) does an aromatic have?
|
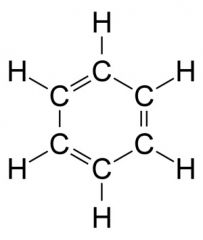
hexagon ring with carbon double bonds on every other carbon
|
|
|
Alcohols
|
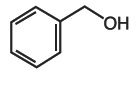
pictured above: benzyl alcohol
|
|
|
What is a feature of an ether?
|
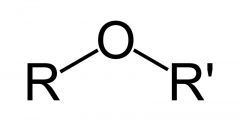
an oxygen atom connected to two alkyl or aryl groups — of general formula R–O–R
|
|
|
What features does an aldehyde have?
|
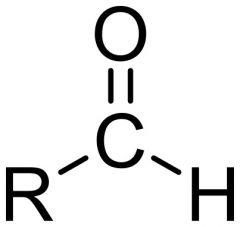
This functional group, with the structure R-CHO, consists of a carbonyl center (a carbon double bonded to oxygen) bonded to hydrogen and an R group,[1] which is any generic alkyl or side chain
|
|
|
What feature does a ketone have?
|
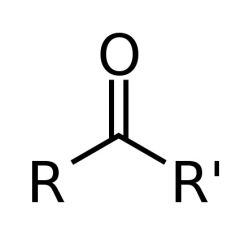
It features a carbonyl group (C=O) bonded to two other carbon atoms
|
|
|
What feature does a carboxylic acid have?
|
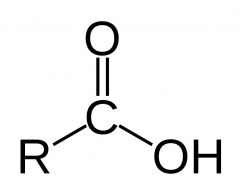
consisting of a carbonyl adjacent to an ether linkage
|
|
|
What feature does an ester have?
|
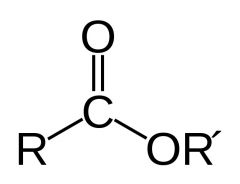
consisting of a carbonyl adjacent to an ether linkage
|
|
|
What is a feature of an amine?
|
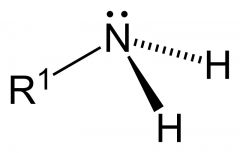
contain a basic nitrogen atom with a lone pair
|
|
|
What is a feature of an amide?
|
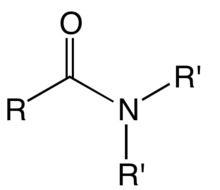
a nitrogen attached to a carbon with a double bonded oxygen present
|
|
|
Alkanes
|
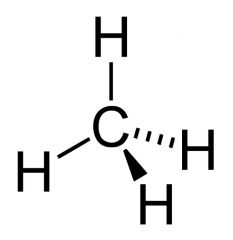
consist only of hydrogen and carbon atoms and are bonded exclusively by single bonds
|
|
|
How to name an alkene:
|
1. Name the alkane
2. Delete the "ane" 3. Replace with "ene" |
|
|
Alkane →(-4H)→ _____
|
Alkynes
|
|
|
How to name an alkyne:
Change CH₃-CH₃ to an alkyne |
1. CH₃-CH₃ is named ethane
2. delete the "ane" 3. replace with "yne" ANSWER is: ethyne 1. Name the alkane 2. Delete the "ane" 3. Replace with "yne" |
|
|
A 6 membered carbon ring with alternate single and double bonds; but all the carbons are identical
|
Aromatic Hydrocarbon
|
|
|
What is a thioalcohol?
|
an alcohol in which the oxygen is replaced with sulfur
|
|
|
What is a primary alcohol?
|
alcohol carbon attached to one carbon and 2 hydrogens
|
|
|
What is a secondary alcohol?
|
alcohol carbon attached to two carbons and one hydrogen
|
|
|
What is a tertiary alcohol?
|
alcohol carbon attached to three carbons and no hydrogens
|
|
|
Loss of hydrogen and a gain of oxygen is an _____.
|
oxidation
|
|
|
A primary alcohol or mild oxidation using [PCC] resutls in an _____.
|
aldehyde
|
|
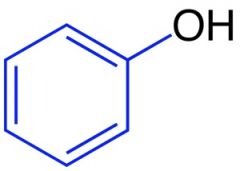
Name this structure
|
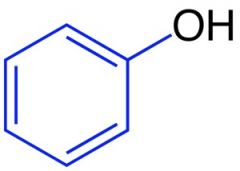
phenol
|
|
|
How do you name an alcohol?
Name CH₃-OH |
CH₃-OH
1. name the alkane - methane 2. delete the "e" - methan 3. replace with "ol" - methanol ANSWER - methanol |
|
|
How to do you name a thioalcohol?
Name CH₃-CH₂CH₂-SH |
1. name the alkane - propane
2. add thiol to the end ANSWER - propane thiol |
|
|
Name this structure
CH₃-O-CH₃ |
dimethyl ether
Why? There are two methyl (dimethyl) and the O in the formula represents an ether |
|
|
Name the structure
CH₃CH₂CH₂-O-CH₂CH₃ |
ethyl propyl ether
WHY? ethyl is from CH₂CH₃, propyl is from CH₃CH₂CH₂ (must be alphabetical so... ethyl before propyl) and the O represents an ether |
|
|
How do you name an aldehyde?
Name CH₃CH₂CH₂CH₂CH₂CH₂CHO |
1. name the alkane
2. delete the "e" 3. replace with "al" ANSWER: heptanal |
|
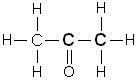
How do you name a ketone?
Name the structure |
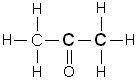
1. Name the alkane
2. delete the "e" 3. add "one" ANSWER: propanone |

